Page 405 - Book Hosokawa Nanoparticle Technology Handbook
P. 405
6.8 CATALYTIC PROPERTY FUNDAMENTALS
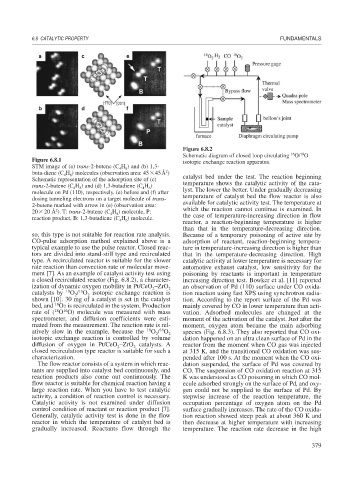
Figure 6.8.2
18
16
Schematic diagram of closed loop circulating O/ O
Figure 6.8.1 isotopic exchange reaction apparatus.
STM image of (a) trans-2-butene (C H ) and (b) 1,3-
8
4
2
buta-diene (C H ) molecules (observation area: 45 45Å )
6
4
Schematic representation of the adsorption site of (c) catalyst bed under the test. The reaction beginning
trans-2-butene (C H ) and (d) 1,3-butadiene (C H ) temperature shows the catalytic activity of the cata-
6
4
4
8
molecule on Pd (110), respectively. (e) before and (f) after lyst. The lower the better. Under gradually decreasing
dosing tunneling electrons on a target molecule of trans- temperature of catalyst bed the flow reactor is also
2-butene marked with arrow in (e) (observation area: available for catalytic activity test. The temperature at
2
20 20 Å ). T: trans-2-butene (C H ) molecule, P: which the reaction cannot continue is examined. In
8
4
reaction product, B: 1,3-butadiene (C H ) molecule. the case of temperature-increasing direction in flow
4
6
reactor, a reaction-beginning temperature is higher
than that in the temperature-decreasing direction.
so, this type is not suitable for reaction rate analysis. Because of a temporary poisoning of active site by
CO-pulse adsorption method explained above is a adsorption of reactant, reaction-beginning tempera-
typical example to use the pulse reactor. Closed reac- ture in temperature-increasing direction is higher than
tors are divided into stand-still type and recirculated that in the temperature-decreasing direction. High
type. A recirculated reactor is suitable for the slower catalytic activity at lower temperature is necessary for
rate reaction than convection rate or molecular move- automotive exhaust catalyst, low sensitivity for the
ment [7]. As an example of catalyst activity test using poisoning by reactants is important in temperature
a closed recirculated reactor (Fig. 6.8.2), a character- increasing direction test. Bowker et al. [11] reported
ization of dynamic oxygen mobility in Pt/CeO –ZrO 2 an observation of Pd (110) surface under CO oxida-
2
16
18
catalysts by O / O isotopic exchange reaction is tion reaction using fast XPS using synchrotron radia-
2
2
shown [10]. 30 mg of a catalyst is set in the catalyst tion. According to the report surface of the Pd was
18
bed, and O2 is recirculated in the system. Production mainly covered by CO in lower temperature than acti-
18
16
rate of ( O O) molecule was measured with mass vation. Adsorbed molecules are changed at the
spectrometer, and diffusion coefficients were esti- moment of the activation of the catalyst. Just after the
mated from the measurement. The reaction rate is rel- moment, oxygen atom became the main adsorbing
18
16
atively slow in the example, because the O / O 2 species (Fig. 6.8.3). They also reported that CO oxi-
2
isotopic exchange reaction is controlled by volume dation happened on an ultra clean surface of Pd in the
diffusion of oxygen in Pt/CeO –ZrO catalysts. A reactor from the moment when CO gas was injected
2
2
closed recirculation type reactor is suitable for such a at 315 K, and the transitional CO oxidation was sus-
characterization. pended after 100 s. At the moment when the CO oxi-
The flow reactor consists of a system in which reac- dation suspended, the surface of Pd was covered by
tants are supplied into catalyst bed continuously, and CO. The suspension of CO oxidation reaction at 315
reaction products also come out continuously. The K was understood as CO poisoning in which CO mol-
flow reactor is suitable for chemical reaction having a ecule adsorbed strongly on the surface of Pd, and oxy-
large reaction rate. When you have to test catalytic gen could not be supplied to the surface of Pd. By
activity, a condition of reaction control is necessary. stepwise increase of the reaction temperature, the
Catalytic activity is not examined under diffusion occupation percentage of oxygen atom on the Pd
control condition of reactant or reaction product [7]. surface gradually increases. The rate of the CO oxida-
Generally, catalytic activity test is done in the flow tion reaction showed steep peak at about 360 K and
reactor in which the temperature of catalyst bed is then decrease at higher temperature with increasing
gradually increased. Reactants flow through the temperature. The reaction rate decrease in the high
379