Page 425 - Book Hosokawa Nanoparticle Technology Handbook
P. 425
7.2 NANOPARTICLES AND ENVIRONMENT FUNDAMENTALS
silicon-containing compounds that precipitated on the (4) Haze on solid surfaces by chemical reaction
electrodes result from the gas-to-particle conversion Haze might form on glass surfaces of lenses and mir-
of low-molecular-weight cyclosiloxane (LMCS) from rors for optical instruments if they are exposed for a
silicone sealant via corona discharge [4]. long time to cleanroom environments where AMCs are
not controlled properly. The haze is more likely to bring
(3) Boron-containing particles from HEPA filter
about the insufficient light delivery onto a surface to be
with borosilicate glass fibers exposed in photolithographic processes. One of the
The use of HEPA and ULPA filters made of borosili- reasons is that ammonium sulfate ((NH ) SO ) is
4 2
4
cate glass fibers prevails in the most cleanrooms. It formed, which then precipitates on the glass surfaces
has been known that owing to chemical reaction in via chemical reaction of sulfur dioxide (SO ) with
2
equation (7.2.2) BF vapor is formed from glass fiber ammonia or amines.
3
filters by passing HF gas leaking from wet cleaning For another reason, hexamethyldisilazane
equipment through the filters. Boron, which is a (HMDS) used as additive in resist coating or LMCS
dopant element for semiconductors, has been thought from silicone sealant is adsorbed, and then decom-
to be a contaminant that might cause failure in semi- posed to form silica precipitates on glass surfaces
conductor devices if it comes from the surroundings. by photochemical reaction during laser irradiation,
In addition, it has been revealed that trace amounts of followed by the unwanted decline in laser penetra-
boron in the form of boric acid (H BO ) are also tion [6]. As another example a report said that tiny
4
3
formed from the fibers via the reaction with moisture projections, which are also known as “haze”, with a
in the surrounding air (equation (7.2.3)). size of 0.2 m or smaller were formed on silicon
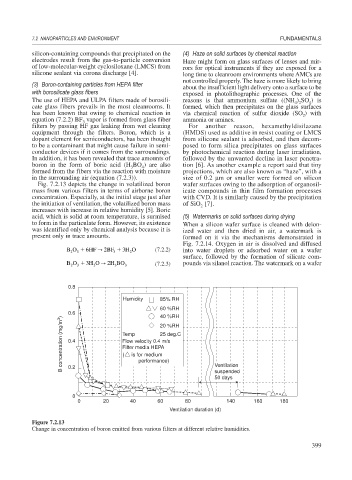
Fig. 7.2.13 depicts the change in volatilized boron wafer surfaces owing to the adsorption of organosil-
mass from various filters in terms of airborne boron icate compounds in thin film formation processes
concentration. Especially, at the initial stage just after with CVD. It is similarly caused by the precipitation
the initiation of ventilation, the volatilized boron mass of SiO [7].
2
increases with increase in relative humidity [5]. Boric
acid, which is solid at room temperature, is surmised (5) Watermarks on solid surfaces during drying
to form in the particulate form. However, its existence When a silicon wafer surface is cleaned with deion-
was identified only by chemical analysis because it is ized water and then dried in air, a watermark is
present only in trace amounts. formed on it via the mechanisms demonstrated in
Fig. 7.2.14. Oxygen in air is dissolved and diffused
BO 6 HF 2 BF 3 H O (7.2.2) into water droplets or adsorbed water on a wafer
2
3
3
2
surface, followed by the formation of silicate com-
BO 3 H O 2 H BO 3 (7.2.3) pounds via silanol reaction. The watermark on a wafer
3
2
3
2
0.8
Humidity 85% RH
60 %RH
0.6 40 %RH
B concentration (mg/m 3 ) 0.4 Temp 25 deg.C
20 %RH
Flow velocity 0.4 m/s
Filter media HEPA
( is for medium
performance)
Ventilation
0.2
suspended
50 days
0
0 20 40 60 80 140 160 180
Ventilation duration (d)
Figure 7.2.13
Change in concentration of boron emitted from various filters at different relative humidities.
399