Page 440 - Book Hosokawa Nanoparticle Technology Handbook
P. 440
FUNDAMENTALS CH. 7 ENVIRONMENTAL AND SAFETY ISSUES WITH NANOPARTICLES
the permeate, and v the fictitious filtrate volume per destabilized by such consideration as depression of the
m
unit membrane area, equivalent to the flow resistance electrical double layer. Thus, the BSA molecules have
of the membrane [4]. water bound to them even around the isoelectric point.
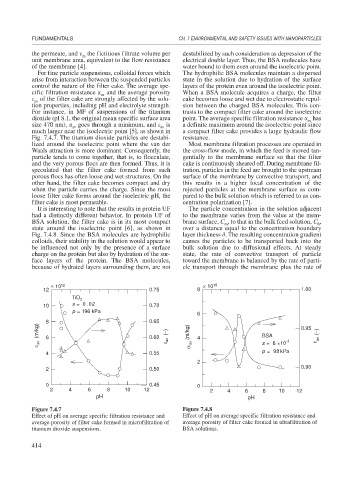
For fine particle suspensions, colloidal forces which The hydrophilic BSA molecules maintain a dispersed
arise from interaction between the suspended particles state in the solution due to hydration of the surface
control the nature of the filter cake. The average spe- layers of the protein even around the isoelectric point.
cific filtration resistance and the average porosity When a BSA molecule acquires a charge, the filter
av
of the filter cake are strongly affected by the solu- cake becomes loose and wet due to electrostatic repul-
av
tion properties, including pH and electrolyte strength. sion between the charged BSA molecules. This con-
For instance, in MF of suspensions of the titanium trasts to the compact filter cake around the isoelectric
dioxide (pI 8.1, the original mean specific surface area point. The average specific filtration resistance has
av
size 470 nm), goes through a minimum, and is a definite maximum around the isoelectric point since
av
av
much larger near the isoelectric point [5], as shown in a compact filter cake provides a large hydraulic flow
Fig. 7.4.7. The titanium dioxide particles are destabi- resistance.
lized around the isoelectric point where the van der Most membrane filtration processes are operated in
Waals attraction is more dominant. Consequently, the the cross-flow mode, in which the feed is moved tan-
particle tends to come together, that is, to flocculate, gentially to the membrane surface so that the filter
and the very porous flocs are then formed. Thus, it is cake is continuously sheared off. During membrane fil-
speculated that the filter cake formed from such tration, particles in the feed are brought to the upstream
porous flocs has often loose and wet structures. On the surface of the membrane by convective transport, and
other hand, the filter cake becomes compact and dry this results in a higher local concentration of the
when the particle carries the charge. Since the most rejected particles at the membrane surface as com-
loose filter cake forms around the isoelectric pH, the pared to the bulk solution which is referred to as con-
filter cake is most permeable. centration polarization [7].
It is interesting to note that the results in protein UF The particle concentration in the solution adjacent
had a distinctly different behavior. In protein UF of to the membrane varies from the value at the mem-
BSA solution, the filter cake is in its most compact brane surface, C , to that in the bulk feed solution, C ,
b
m
state around the isoelectric point [6], as shown in over a distance equal to the concentration boundary
Fig. 7.4.8. Since the BSA molecules are hydrophilic layer thickness . The resulting concentration gradient
colloids, their stability in the solution would appear to causes the particles to be transported back into the
be influenced not only by the presence of a surface bulk solution due to diffusional effects. At steady
charge on the protein but also by hydration of the sur- state, the rate of convective transport of particle
face layers of the protein. The BSA molecules, toward the membrane is balanced by the rate of parti-
because of hydrated layers surrounding them, are not cle transport through the membrane plus the rate of
× 10 12 × 10 15
12 0.75 8 1.00
TiO 2
10 s =0 .02 0.70
p = 196 kPa
6
8 0.65
(m/kg) 6 0.60 (−) α av (m/kg) 4 BSA 0.95 (−)
av av s =6 ×10 -3 ε av
4 0.55 p =98 kPa
2
0.90
2 0.50
0 0.45 0
2 4 6 8 10 12 2 4 6 8 10 12
pH pH
Figure 7.4.7 Figure 7.4.8
Effect of pH on average specific filtration resistance and Effect of pH on average specific filtration resistance and
average porosity of filter cake formed in microfiltration of average porosity of filter cake formed in ultrafiltration of
titanium dioxide suspensions. BSA solutions.
414