Page 487 - Book Hosokawa Nanoparticle Technology Handbook
P. 487
9 DEVELOPMENT OF POLYMER-CLAY NANOCOMPOSITES APPLICATIONS
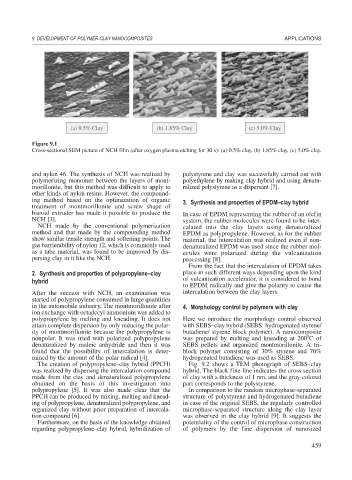
(a) 0.5% Clay (b) 1.85% Clay (c) 5.0% Clay
Figure 9.1
Cross-sectional SEM picture of NCH film (after oxygen plasma etching for 30 s): (a) 0.5% clay, (b) 1.85% clay, (c) 5.0% clay.
and nylon 46. The synthesis of NCH was realized by polystyrene and clay was successfully carried out with
polymerizing monomer between the layers of mont- polyethylene by making clay hybrid and using denatu-
morillonite, but this method was difficult to apply to ralized polystyrene as a dispersant [7].
other kinds of nylon resins. However, the compound-
ing method based on the optimization of organic 3. Synthesis and properties of EPDM–clay hybrid
treatment of montmorillonite and screw shape of
biaxial extruder has made it possible to produce the In case of EPDM representing the rubber of an olefin
NCH [3]. system, the rubber molecules were found to be inter-
NCH made by the conventional polymerization calated into the clay layers using denaturalized
method and that made by the compounding method EPDM as polypropylene. However, as for the rubber
show similar tensile strength and softening points. The material, the intercalation was realized even if non-
gas barrierability of nylon 12, which is commonly used denaturalized EPDM was used since the rubber mol-
as a tube material, was found to be improved by dis- ecules were polarized during the vulcanization
persing clay in it like the NCH. processing [8].
From the fact that the intercalation of EPDM takes
2. Synthesis and properties of polypropylene–clay place in such different ways depending upon the kind
of vulcanization accelerator, it is considered to bond
hybrid
to EPDM radically and give the polarity to cause the
After the success with NCH, an examination was intercalation between the clay layers.
started of polypropylene consumed in large quantities
in the automobile industry. The montmorillonite after 4. Morphology control by polymers with clay
ion exchange with octadecyl ammonium was added to
polypropylene by melting and kneading. It does not Here we introduce the morphology control observed
attain complete dispersion by only reducing the polar- with SEBS–clay hybrid (SEBS: hydrogenated styrene/
ity of montmorillonite because the polypropylene is butadiene/ styrene block polymer). A nanocomposite
nonpolar. It was tried with polarized polypropylene was prepared by melting and kneading at 200 C of
denaturalized by maleic anhydride and then it was SEBS pellets and organized montmorillonite. A tri-
found that the possibility of intercalation is deter- block polymer consisting of 30% styrene and 70%
mined by the amount of the polar radical [4]. hydrogenated butadiene was used as SEBS.
The creation of polypropylene–clay hybrid (PPCH) Fig. 9.2 shows a TEM photograph of SEBS–clay
was realized by dispersing the intercalation compound hybrid. The black fine line indicates the cross section
made from the clay and denaturalized polypropylene of clay with a thickness of 1 nm, and the gray-colored
obtained on the basis of this investigation into part corresponds to the polystyrene.
polypropylene [5]. It was also made clear that the In comparison to the random microphase-separated
PPCH can be produced by mixing, melting and knead- structure of polystyrene and hydrogenated butadiene
ing of polypropylene, denaturalized polypropylene, and in case of the original SEBS, the regularly controlled
organized clay without prior preparation of intercala- microphase-separated structure along the clay layer
tion compound [6]. was observed in the clay hybrid [9]. It suggests the
Furthermore, on the basis of the knowledge obtained potentiality of the control of microphase construction
regarding polypropylene–clay hybrid, hybridization of of polymers by the fine dispersion of nanosized
459