Page 558 - Book Hosokawa Nanoparticle Technology Handbook
P. 558
APPLICATIONS 26 DISPERSION CONTROL OF Al O NANOPARTICLES IN ETHANOL
2 3
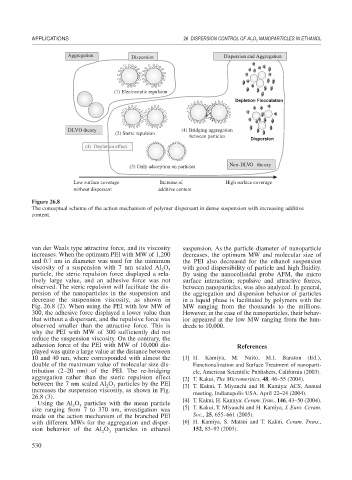
Aggregation Dispersion Dispersion and Aggregation
(1) Electrostatic repulsion
Depletion Flocculation
DLVO theory (4) Bridging aggregation
(2) Steric repulsion
between particles
Dispersion
(4) Depletion effect
Non-DLVO theory
(3) Only adsorption on particles
Low surface coverage Increase of High surface coverage
without dispersant additive content
Figure 26.8
The conceptual scheme of the action mechanism of polymer dispersant in dense suspension with increasing additive
content.
van der Waals type attractive force, and its viscosity suspension. As the particle diameter of nanoparticle
increases. When the optimum PEI with MW of 1,200 decreases, the optimum MW and molecular size of
and 0.7 nm in diameter was used for the minimum the PEI also decreased for the ethanol suspension
viscosity of a suspension with 7 nm scaled Al O 3 with good dispersibility of particle and high fluidity.
2
particle, the steric repulsion force displayed a rela- By using the nanocolloidal probe AFM, the micro
tively large value, and an adhesive force was not surface interaction; repulsive and attractive forces,
observed. The steric repulsion will facilitate the dis- between nanoparticles, was also analyzed. In general,
persion of the nanoparticles in the suspension and the aggregation and dispersion behavior of particles
decrease the suspension viscosity, as shown in in a liquid phase is facilitated by polymers with the
Fig. 26.8 (2). When using the PEI with low MW of MW ranging from the thousands to the millions.
300, the adhesive force displayed a lower value than However, in the case of the nanoparticles, their behav-
that without a dispersant, and the repulsive force was ior appeared at the low MW ranging from the hun-
observed smaller than the attractive force. This is dreds to 10,000.
why the PEI with MW of 300 sufficiently did not
reduce the suspension viscosity. On the contrary, the
adhesion force of the PEI with MW of 10,000 dis- References
played was quite a large value at the distance between
10 and 40 nm, where corresponded with almost the [1] H. Kamiya, M. Naito, M.I. Baraton (Ed.),
double of the maximum value of molecular size dis- Functionalization and Surface Treatment of nanoparti-
tribution (2–20 nm) of the PEI. The re-bridging cle, American Scientific Publishers, California (2003).
aggregation rather than the steric repulsion effect [2] T. Kakui, The Micromeritics, 48, 46–55 (2004).
between the 7 nm scaled Al O particles by the PEI [3] T. Kakui, T. Miyauchi and H. Kamiya: ACS, Annual
2
3
increases the suspension viscosity, as shown in Fig. meeting, Indianapolis USA, April 22–24 (2004).
26.8 (3). [4] T. Kakui, H. Kamiya: Ceram. Tran., 146, 43–50 (2004).
Using the Al O particles with the mean particle
3
2
size ranging from 7 to 370 nm, investigation was [5] T. Kakui, T. Miyauchi and H. Kamiya, J. Euro. Ceram.
made on the action mechanism of the branched PEI Soc., 25, 655–661 (2005).
with different MWs for the aggregation and disper- [6] H. Kamiya, S. Matsui and T. Kakui, Ceram. Trans.,
sion behavior of the Al O particles in ethanol 152, 83–92 (2003).
3
2
530