Page 560 - Book Hosokawa Nanoparticle Technology Handbook
P. 560
APPLICATIONS 27 DEVELOPMENT OF THE THERMORESPONSIVE MAGNETIC NANOPARTICLE
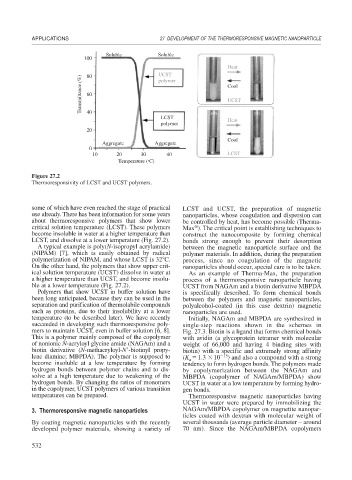
100 Soluble Soluble
Heat
80
UCST
Transmittance (%) 60 Cool
polymer
UCST
40
LCST
Heat
polymer
20
Cool
Aggregate Aggregate
0
10 20 30 40 LCST
Temperature (°C)
Figure 27.2
Thermoresponsivity of LCST and UCST polymers.
some of which have even reached the stage of practical LCST and UCST, the preparation of magnetic
use already. There has been information for some years nanoparticles, whose coagulation and dispersion can
about thermoresponsive polymers that show lower be controlled by heat, has become possible (Therma-
critical solution temperature (LCST). These polymers Max ). The critical point is establishing techniques to
®
become insoluble in water at a higher temperature than construct the nanocomposite by forming chemical
LCST, and dissolve at a lower temperature (Fig. 27.2). bonds strong enough to prevent their desorption
A typical example is poly(N-isopropyl acrylamide) between the magnetic nanoparticle surface and the
(NIPAM) [7], which is easily obtained by radical polymer materials. In addition, during the preparation
polymerization of NIPAM, and whose LCST is 32 C. process, since no coagulation of the magnetic
On the other hand, the polymers that show upper crit- nanoparticles should occur, special care is to be taken.
ical solution temperature (UCST) dissolve in water at As an example of Therma-Max, the preparation
a higher temperature than UCST, and become insolu- process of a thermoresponsive nanoparticle having
ble at a lower temperature (Fig. 27.2). UCST from NAGAm and a biotin derivative MBPDA
Polymers that show UCST in buffer solution have is specifically described. To form chemical bonds
been long anticipated, because they can be used in the between the polymers and magnetic nanoparticles,
separation and purification of thermolabile compounds polyalcohol-coated (in this case dextrin) magnetic
such as proteins, due to their insolubility at a lower nanoparticles are used.
temperature (to be described later). We have recently Initially, NAGAm and MBPDA are synthesized in
succeeded in developing such thermoresponsive poly- single-step reactions shown in the schemes in
mers to maintain UCST, even in buffer solution [6, 8]. Fig. 27.3. Biotin is a ligand that forms chemical bonds
This is a polymer mainly composed of the copolymer with avidin (a glycoprotein tetramer with molecular
of nonionic N-acryloyl glycine amide (NAGAm) and a weight of 66,000 and having 4 binding sites with
biotin derivative (N-methacryloyl-N
-biotinyl propy- biotin) with a specific and extremely strong affinity
lene diamine; MBPDA). The polymer is supposed to (K 1.3 10 15 ) and also a compound with a strong
a
become insoluble at a low temperature by forming tendency to form hydrogen bonds. The polymers made
hydrogen bonds between polymer chains and to dis- by copolymerization between the NAGAm and
solve at a high temperature due to weakening of the MBPDA (copolymer of NAGAm/MBPDA) show
hydrogen bonds. By changing the ratios of monomers UCST in water at a low temperature by forming hydro-
in the copolymer, UCST polymers of various transition gen bonds.
temperatures can be prepared. Thermoresponsive magnetic nanoparticles having
UCST in water were prepared by immobilizing the
3. Thermoresponsive magnetic nanoparticles NAGAm/MBPDA copolymer on magnetite nanopar-
ticles coated with dextran with molecular weight of
By coating magnetic nanoparticles with the recently several thousands (average particle diameter – around
developed polymer materials, showing a variety of 70 nm). Since the NAGAm/MBPDA copolymers
532