Page 625 - Book Hosokawa Nanoparticle Technology Handbook
P. 625
42 FABRICATION TECHNIQUE OF ORGANIC NANOCRYSTALS APPLICATIONS
R
R
N +
N −
hv or D
R R R′ SO 3 − C 60
R′
DAST
N N
R′ R′ N O
Monomer Polymer N
Ti N
N N N
TPB
− TiOPc
DCHD: R=R= CH N
2
O
H
N R
Polydiacetylene
derivatives R N
H
O
Perylene
R = H or CH 3
Quinacridone
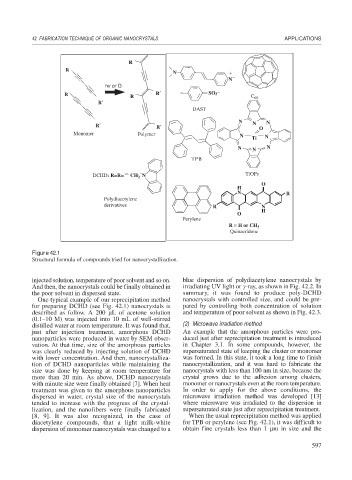
Figure 42.1
Structural formula of compounds tried for nanocrystallization.
injected solution, temperature of poor solvent and so on. blue dispersion of polydiacetylene nanocrystals by
And then, the nanocrystals could be finally obtained in irradiating UV light or -ray, as shown in Fig. 42.2. In
the poor solvent in dispersed state. summary, it was found to produce poly-DCHD
One typical example of our reprecipitation method nanocrystals with controlled size, and could be pre-
for preparing DCHD (see Fig. 42.1) nanocrystals is pared by controlling both concentration of solution
described as follow. A 200 L of acetone solution and temperature of poor solvent as shown in Fig. 42.3.
(0.1–10 M) was injected into 10 mL of well-stirred
distilled water at room temperature. It was found that, (2) Microwave irradiation method
just after injection treatment, amorphous DCHD An example that the amorphous particles were pro-
nanoparticles were produced in water by SEM obser- duced just after reprecipitation treatment is introduced
vation. At that time, size of the amorphous particles in Chapter 3.1. In some compounds, however, the
was clearly reduced by injecting solution of DCHD supersaturated state of keeping the cluster or monomer
with lower concentration. And then, nanocrystalliza- was formed. In this state, it took a long time to finish
tion of DCHD nanoparticles while maintaining the nanocrystallization, and it was hard to fabricate the
size was done by keeping at room temperature for nanocrystals with less than 100 nm in size, because the
more than 20 min. As above, DCHD nanocrystals crystal grows due to the adhesion among clusters,
with minute size were finally obtained [7]. When heat monomer or nanocrystals even at the room temperature.
treatment was given to the amorphous nanoparticles In order to apply for the above conditions, the
dispersed in water, crystal size of the nanocrystals microwave irradiation method was developed [13]
tended to increase with the progress of the crystal- where microwave was irradiated to the dispersion in
lization, and the nanofibers were finally fabricated supersaturated state just after reprecipitation treatment.
[8, 9]. It was also recognized, in the case of When the usual reprecipitation method was applied
diacetylene compounds, that a light milk-white for TPB or perylene (see Fig. 42.1), it was difficult to
dispersion of monomer nanocrystals was changed to a obtain fine crystals less than 1 m in size and the
597