Page 82 - New Trends In Coal Conversion
P. 82
48 New Trends in Coal Conversion
cleanup. The process operates at high temperature and pressure: 350e500 C and
10e50 bar. Moreover, SEWGS maximizes H 2 production by the simultaneous
removal of CO 2 . Potassium-promoted hydrotalcite presents high CO 2 capture capacity
at 400 C, catalytic activity for the WGS reaction, and excellent chemical and mechan-
þ
ical stability, being an ideal candidate for SEWGS. ALKASORB sorbent was devel-
oped in the framework of the European FP7 CAESAR project. SEWGS has been
demonstrated at bench scale (25 kW th ) with technical gases in a unit with 6 reactors
at the Energy Research Centre of the Netherlands. The results were used to validate
a simulation model that was later used to evaluate the upscale of the process to a
full IGCC plant. The resulting cost, 23 V/t CO 2 , for a capture rate of 90%, is consid-
erably lower than the Selexol case. The estimated energy consumption for this process
is 2.1 GJ/t CO 2 (Jansen et al., 2013).
2.5 CO capture in coal-based industrial processes
2
2.5.1 CO 2 capture in coal-to-chemicals industry
Coal syngas is used as feedstock for the manufacture of ammonia, methanol, Fischere
Tropsch, oxo alcohols, H 2 , reduction gas, and town gas in the coal-to-chemicals (CTC)
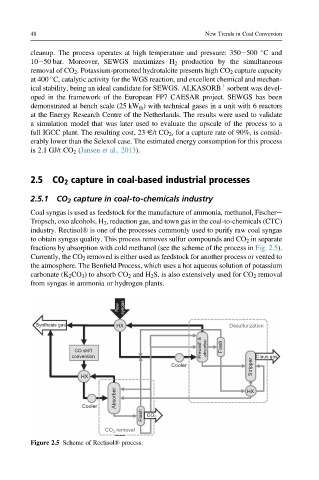
industry. Rectisol® is one of the processes commonly used to purify raw coal syngas
to obtain syngas quality. This process removes sulfur compounds and CO 2 in separate
fractions by absorption with cold methanol (see the scheme of the process in Fig. 2.5).
Currently, the CO 2 removed is either used as feedstock for another process or vented to
the atmosphere. The Benfield Process, which uses a hot aqueous solution of potassium
carbonate (K 2 CO 3 ) to absorb CO 2 and H 2 S, is also extensively used for CO 2 removal
from syngas in ammonia or hydrogen plants.
Raw syngas
Synthesis gas HX Desulfurization
Prewash & absorber Flash
CO shift
conversion
Stripper Claus gas
Cooler
HX
Absorber HX
Cooler
Flash CO 2
CO 2 removal
Figure 2.5 Scheme of Rectisol® process.