Page 26 - Organic Electronics in Sensors and Biotechnology
P. 26
Scaling Effects in Organic Transistors and Transistor-Based Chemical Sensors 3
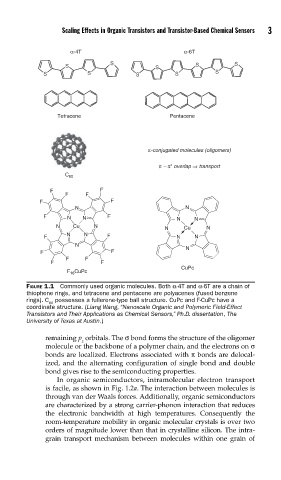
α-4T α-6T
S S
S S S
S S S S S
Tetracene Pentacene
π-conjugated molecules (oligomers)
π – π* overlap ⇒ transport
C 60
F F
F F
F F
N N
F N N F N N
N Cu N
N Cu N
F N N F N N
N N
F F
F F
F F
CuPc
F 16 CuPc
FIGURE 1.1 Commonly used organic molecules. Both α-4T and α-6T are a chain of
thiophene rings, and tetracene and pentacene are polyacenes (fused benzene
rings). C possesses a fullerene-type ball structure. CuPc and F-CuPc have a
60
coordinate structure. (Liang Wang, “Nanoscale Organic and Polymeric Field-Effect
Transistors and Their Applications as Chemical Sensors,” Ph.D. dissertation, The
University of Texas at Austin.)
remaining p orbitals. The σ bond forms the structure of the oligomer
z
molecule or the backbone of a polymer chain, and the electrons on σ
bonds are localized. Electrons associated with π bonds are delocal-
ized, and the alternating configuration of single bond and double
bond gives rise to the semiconducting properties.
In organic semiconductors, intramolecular electron transport
is facile, as shown in Fig. 1.2a. The interaction between molecules is
through van der Waals forces. Additionally, organic semiconductors
are characterized by a strong carrier-phonon interaction that reduces
the electronic bandwidth at high temperatures. Consequently the
room-temperature mobility in organic molecular crystals is over two
orders of magnitude lower than that in crystalline silicon. The intra-
grain transport mechanism between molecules within one grain of