Page 387 - Organic Electronics in Sensors and Biotechnology
P. 387
364 Chapter Ten
Electrophoretic
Property Electroplating Deposition
Moving species Ions Solid particles
Charge transfer on Ion reduction None
deposition
Required conductance of High Low
liquid medium
Preferred liquid Water Organic
TABLE 10.1 Comparison between EPD and Electroplating
electrodeposition is often ambiguously used to refer to either electro-
plating or EPD, although it more often refers to the former. Table 10.1
presents the difference between the two processes.
EPD can be of two types depending upon the electrode at which the
deposition occurs. When the particles are positively charged, then the
deposition occurs on the cathode (negatively biased electrode) and
the process is called as cathodic EPD or cataphoresis. Deposition of neg-
atively charged particles on the anode (positively biased electrode) is
known as anodic EPD or anaphoresis. Either of the two modes of deposi-
tion can be used by suitable modification of the surface charge on the
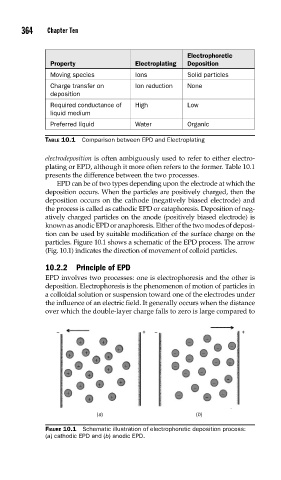
particles. Figure 10.1 shows a schematic of the EPD process. The arrow
(Fig. 10.1) indicates the direction of movement of colloid particles.
10.2.2 Principle of EPD
EPD involves two processes: one is electrophoresis and the other is
deposition. Electrophoresis is the phenomenon of motion of particles in
a colloidal solution or suspension toward one of the electrodes under
the influence of an electric field. It generally occurs when the distance
over which the double-layer charge falls to zero is large compared to
– + – +
–
+ + –
– –
+
+ – –
+ +
+ – –
+ + – –
+
+ + – –
–
+ –
+ +
–
+ – –
+ + –
(a) (b)
FIGURE 10.1 Schematic illustration of electrophoretic deposition process:
(a) cathodic EPD and (b) anodic EPD.