Page 389 - Organic Electronics in Sensors and Biotechnology
P. 389
366 Chapter Ten
+ –
+ – – + – + +
– + + – + + – – +
+ – Distortion of – – – –
–
– – – – – – – – + Iyosphere by EPD – + + – – – – – – – – –
+ – + – – – + –– – – – + – –
– – – – – – – + – – – – –
+ – – + + + +
+ – + + – + + +
– – –
+ – + –
– + + – +
+ + – – + Lyosphere + +
– – thinning –
+ – – – – – – + – – – – –
–
– + – – – – – + – +
–
+ –– – – – + – – + – – – – –
– + – – – – – –
+ + + +
+ + + –
– –
+ – + –
+ +
– + + Lyosphere – + – +
– – – – – – – – – – – – coagulation + – – – – –
– – – + +
– – – + – – – – + – – – – – – –
– – – – – –
+ + + +
+ + + +
– –
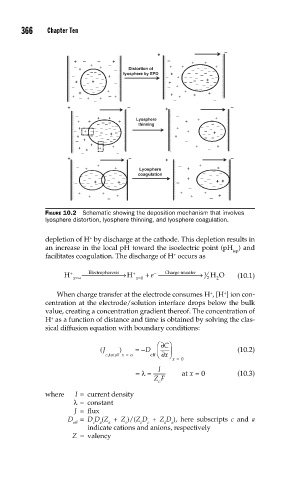
FIGURE 10.2 Schematic showing the deposition mechanism that involves
lyosphere distortion, lyosphere thinning, and lyosphere coagulation.
+
depletion of H by discharge at the cathode. This depletion results in
an increase in the local pH toward the isoelectric point (pH ) and
iep
+
facilitates coagulation. The discharge of H occurs as
→
Charge traansfer
−
→
Electrophoresis
H + x=∞ ⎯⎯⎯⎯⎯⎯ H + x=0 + e ⎯⎯⎯⎯⎯⎯ 1 2 HO (10.1)
2
When charge transfer at the electrode consumes H , [H ] ion con-
+
+
centration at the electrode/solution interface drops below the bulk
value, creating a concentration gradient thereof. The concentration of
+
H as a function of distance and time is obtained by solving the clas-
sical diffusion equation with boundary conditions:
⎛ ∂ C⎞
(J ) =− D ⎜ ⎟ (10.2)
c,total x = o eff ⎝ x ∂ ⎠
x = 0
= λ = I at x = 0 (10.3)
ZF
c
where I = current density
λ= constant
J = flux
D = D D (Z + Z )/(Z D + Z D ), here subscripts c and a
eff c a a c c c a a
indicate cations and anions, respectively
Z = valency