Page 394 - Organic Electronics in Sensors and Biotechnology
P. 394
Electrophoretically Deposited Polymers for Organic Electronics 371
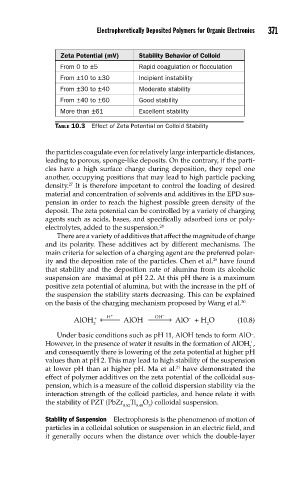
Zeta Potential (mV) Stability Behavior of Colloid
From 0 to ±5 Rapid coagulation or flocculation
From ±10 to ±30 Incipient instability
From ±30 to ±40 Moderate stability
From ±40 to ±60 Good stability
More than ±61 Excellent stability
TABLE 10.3 Effect of Zeta Potential on Colloid Stability
the particles coagulate even for relatively large interparticle distances,
leading to porous, sponge-like deposits. On the contrary, if the parti-
cles have a high surface charge during deposition, they repel one
another, occupying positions that may lead to high particle packing
density. It is therefore important to control the loading of desired
27
material and concentration of solvents and additives in the EPD sus-
pension in order to reach the highest possible green density of the
deposit. The zeta potential can be controlled by a variety of charging
agents such as acids, bases, and specifically adsorbed ions or poly-
electrolytes, added to the suspension. 28
There are a variety of additives that affect the magnitude of charge
and its polarity. These additives act by different mechanisms. The
main criteria for selection of a charging agent are the preferred polar-
ity and the deposition rate of the particles. Chen et al. have found
29
that stability and the deposition rate of alumina from its alcoholic
suspension are maximal at pH 2.2. At this pH there is a maximum
positive zeta potential of alumina, but with the increase in the pH of
the suspension the stability starts decreasing. This can be explained
on the basis of the charging mechanism proposed by Wang et al. 30
−
AlOH ←⎯⎯ + AlOH ⎯⎯⎯ AlO + H O (10.8)
OH
→
H
+
–
2 2
Under basic conditions such as pH 11, AlOH tends to form AlO .
–
+
However, in the presence of water it results in the formation of AlOH ,
2
and consequently there is lowering of the zeta potential at higher pH
values than at pH 2. This may lead to high stability of the suspension
at lower pH than at higher pH. Ma et al. have demonstrated the
31
effect of polymer additives on the zeta potential of the colloidal sus-
pension, which is a measure of the colloid dispersion stability via the
interaction strength of the colloid particles, and hence relate it with
the stability of PZT (PbZr Ti O ) colloidal suspension.
0.52 0.48 3
Stability of Suspension Electrophoresis is the phenomenon of motion of
particles in a colloidal solution or suspension in an electric field, and
it generally occurs when the distance over which the double-layer