Page 420 - Organic Electronics in Sensors and Biotechnology
P. 420
Electrochemical Surface Switches and Electronic Ion Pumps Based on Conjugated Polymers 397
In the oxidized state, the P3HT film exhibits a water contact angle
of θ = 89.1º (Fig. 11.1). In both oxidation states, the water contact
angles are rather high and the associated difference is small. This is
explained by the presence of hexyl side chains. Along the P3HT sur-
face, the side groups point outward to a great extent. Therefore, they
shield the net effect of the dynamic change of the dipole characteris-
tics that occur along the core of the polythiophene backbone, upon
EC switching.
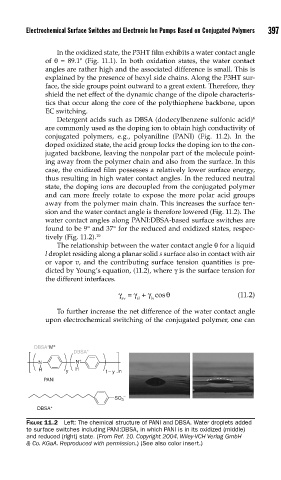
Detergent acids such as DBSA (dodecylbenzene sulfonic acid) 6
are commonly used as the doping ion to obtain high conductivity of
conjugated polymers, e.g., polyaniline (PANI) (Fig. 11.2). In the
doped oxidized state, the acid group locks the doping ion to the con-
jugated backbone, leaving the nonpolar part of the molecule point-
ing away from the polymer chain and also from the surface. In this
case, the oxidized film possesses a relatively lower surface energy,
thus resulting in high water contact angles. In the reduced neutral
state, the doping ions are decoupled from the conjugated polymer
and can more freely rotate to expose the more polar acid groups
away from the polymer main chain. This increases the surface ten-
sion and the water contact angle is therefore lowered (Fig. 11.2). The
water contact angles along PANI:DBSA-based surface switches are
found to be 9° and 37° for the reduced and oxidized states, respec-
tively (Fig. 11.2). 10
The relationship between the water contact angle θ for a liquid
l droplet residing along a planar solid s surface also in contact with air
or vapor v, and the contributing surface tension quantities is pre-
dicted by Young’s equation, (11.2), where γ is the surface tension for
the different interfaces.
γ = γ + γ cosθ (11.2)
sv sl lv
To further increase the net difference of the water contact angle
upon electrochemical switching of the conjugated polymer, one can
DBSA*M +
DBSA*
N N +
H y H 1– y n
PANI
–
SO 3
DBSA*
FIGURE 11.2 Left: The chemical structure of PANI and DBSA. Water droplets added
to surface switches including PANI:DBSA, in which PANI is in its oxidized (middle)
and reduced (right) state. (From Ref. 10. Copyright 2004, Wiley-VCH Verlag GmbH
& Co. KGaA. Reproduced with permission.) (See also color insert.)