Page 419 - Organic Electronics in Sensors and Biotechnology
P. 419
396 Chapter Eleven
static characteristics for the target application. However, for many
bioapplications, it may be preferable to achieve dynamic control of the
chemical and physical surface properties. Dynamic control of the sur-
face tension, chemistry, and charge achieved by electric biasing will
provide a novel technology with great potential to advance current
research in cell biology.
11.1.1 Wettability Switches Based on Conducting Polymers
Conjugated polymers have been extensively explored in electrochemi-
cal (EC) devices. Their principle of operation is defined by the dynamic
change of the fundamental chemical and/or physical properties of the
conjugated polymer bulk upon EC switching. For instance, EC control
of the volume, optical absorption, and impedance define the function
3
2
of polymer actuators, electrochromic displays, and EC transistors. 4,5
As the oxidation state of a polymer film is altered, not only its bulk
properties are switched but also its nature along its outermost surface.
Several research groups have explored the use of EC switching of con-
jugated polymer thin films to achieve dynamic control of the surface
6–8
tension. The surface of an oxidized polymer film expresses a higher
density of dipoles compared to the neutral polymer surface, and intui-
tively, it should therefore exhibit a relatively higher surface energy.
However, wettability along conjugated polymer surfaces is somewhat
more complex, since topography and the properties of doping ions
must be taken into the account.
11.1.2 Surface Switches Based on P3AT, PPy, and PANI
9
Poly(3-alkylthiophenes) (P3AT) can be processed and patterned from
organic solvents. Films of poly(3-hexylthiophene) (P3HT) can be
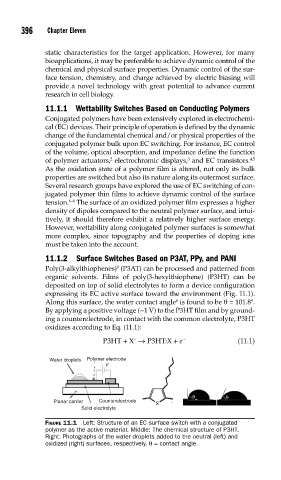
deposited on top of solid electrolytes to form a device configuration
expressing its EC active surface toward the environment (Fig. 11.1).
8
Along this surface, the water contact angle is found to be θ = 101.8º.
By applying a positive voltage (~1 V) to the P3HT film and by ground-
ing a counterelectrode, in contact with the common electrolyte, P3HT
oxidizes according to Eq. (11.1):
−
P3HT + X → P3HT:X + e − (11.1)
Water droplets Polymer electrode
V
θ θ
Planar carrier Counterelectrode
S
Solid electrolyte
FIGURE 11.1 Left: Structure of an EC surface switch with a conjugated
polymer as the active material. Middle: The chemical structure of P3HT.
Right: Photographs of the water droplets added to the neutral (left) and
oxidized (right) surfaces, respectively. θ = contact angle.