Page 87 - Organic Electronics in Sensors and Biotechnology
P. 87
64 Cha pte r T w o
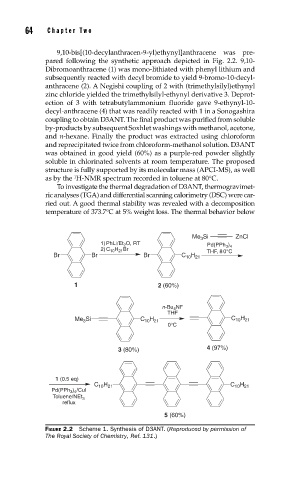
9,10-bis[(10-decylanthracen-9-yl)ethynyl]anthracene was pre-
pared following the synthetic approach depicted in Fig. 2.2. 9,10-
Dibromoanthracene (1) was mono-lithiated with phenyl lithium and
subsequently reacted with decyl bromide to yield 9-bromo-10-decyl-
anthracene (2). A Negishi coupling of 2 with (trimethylsilyl)ethynyl
zinc chloride yielded the trimethylsilyl-ethynyl derivative 3. Deprot-
ection of 3 with tetrabutylammonium fluoride gave 9-ethynyl-10-
decyl-anthracene (4) that was readily reacted with 1 in a Sonogashira
coupling to obtain D3ANT. The final product was purified from soluble
by-products by subsequent Soxhlet washings with methanol, acetone,
and n-hexane. Finally the product was extracted using chloroform
and reprecipitated twice from chloroform-methanol solution. D3ANT
was obtained in good yield (60%) as a purple-red powder slightly
soluble in chlorinated solvents at room temperature. The proposed
structure is fully supported by its molecular mass (APCI-MS), as well
1
as by the H-NMR spectrum recorded in toluene at 80°C.
To investigate the thermal degradation of D3ANT, thermogravimet-
ric analyses (TGA) and differential scanning calorimetry (DSC) were car-
ried out. A good thermal stability was revealed with a decomposition
temperature of 373.7°C at 5% weight loss. The thermal behavior below
Me 3 Si ZnCl
1) P hLi/Et 2 O, R T Pd(PPh 3 ) 4
2) C 10 H 21 Br THF, 8 0°C
Br Br Br C 10 H 21
1 2 (60%)
n-B u 4 NF
THF
Me 3 Si C 10 H 21 C 10 H 21
0°C
3 (80%) 4 (97%)
1 (0. 5 e q)
C 10 H 21 C 10 H 21
Pd(PPh 3 ) 4 /CuI
Toluene/NEt 3
reflux
5 (60%)
FIGURE 2.2 Scheme 1. Synthesis of D3ANT. (Reproduced by permission of
The Royal Society of Chemistry, Ref. 131.)