Page 138 - Origin and Prediction of Abnormal Formation Pressures
P. 138
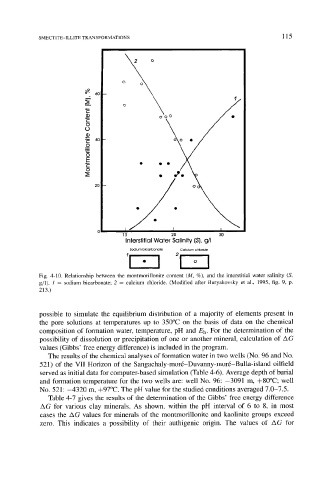
SMECTITE-ILLITE TRANSFORMATIONS 1 15
2 0
o 0
~ 6O
,e--
C"
0
o
9 40
~
0
.~-.
0
E
.I,-.
C"
O
10 20 30
Interstitial Water Salinity (S), g/I
Sodium bicarbonate Calcium chloride
1 2
Fig. 4-10. Relationship between the montmorillonite content (M, %), and the interstitial water salinity (S,
g/l). 1 = sodium bicarbonate; 2 = calcium chloride. (Modified after Buryakovsky et al., 1995, fig. 9, p.
213.)
possible to simulate the equilibrium distribution of a majority of elements present in
the pore solutions at temperatures up to 350~ on the basis of data on the chemical
composition of formation water, temperature, pH and Eh. For the determination of the
possibility of dissolution or precipitation of one or another mineral, calculation of A G
values (Gibbs' free energy difference) is included in the program.
The results of the chemical analyses of formation water in two wells (No. 96 and No.
521) of the VII Horizon of the Sangachaly-mor6-Duvanny-mor6-Bulla-island oilfield
served as initial data for computer-based simulation (Table 4-6). Average depth of burial
and formation temperature for the two wells are: well No. 96:-3091 m, +80~ well
No. 521:-4320 m, +97~ The pH value for the studied conditions averaged 7.0-7.5.
Table 4-7 gives the results of the determination of the Gibbs' free energy difference
A G for various clay minerals. As shown, within the pH interval of 6 to 8, in most
cases the A G values for minerals of the montmorillonite and kaolinite groups exceed
zero. This indicates a possibility of their authigenic origin. The values of A G for