Page 139 - Origin and Prediction of Abnormal Formation Pressures
P. 139
116 L.A. BURYAKOVSKY, R.D. DJEVANSHIR, G.V. CHILINGAR, H.H. RIEKE III AND J.O. ROBERTSON, JR.
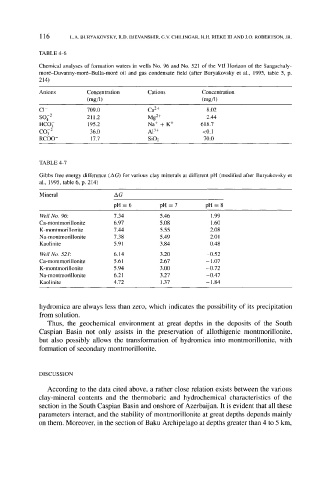
TABLE 4-6
Chemical analyses of formation waters in wells No. 96 and No. 521 of the VII Horizon of the Sangachaly-
mor6-Duvanny-mor6-Bulla-mor6 oil and gas condensate field (after Buryakovsky et al., 1995, table 5, p.
214)
Anions Concentration Cations Concentration
(mg/1) (mg/l)
C1- 709.0 Ca 2+ 8.02
8042 211.2 Mg 2+ 2.44
HCO 3 195.2 Na + + K + 618.7
CO32 36.0 A13+ <0.1
RCOO- 17.7 SiO2 70.0
TABLE 4-7
Gibbs free energy difference (AG) for various clay minerals at different pH (modified after Buryakovsky et
al., 1995, table 6, p. 214)
Mineral AG
pH = 6 pH -- 7 pH -- 8
Well No. 96: 7.34 5.46 1.99
Ca-montmorillonite 6.97 5.08 1.60
K-montmorillonite 7.44 5.55 2.08
Na-montmorillonite 7.38 5.49 2.01
Kaolinite 5.91 3.84 0.48
Well No. 521: 6.14 3.20 -0.52
Ca-montmorillonite 5.61 2.67 - 1.07
K-montmorillonite 5.94 3.00 -0.72
Na-montmorillonite 6.21 3.27 -0.47
Kaolinite 4.72 1.37 - 1.84
hydromica are always less than zero, which indicates the possibility of its precipitation
from solution.
Thus, the geochemical environment at great depths in the deposits of the South
Caspian Basin not only assists in the preservation of allothigenic montmorillonite,
but also possibly allows the transformation of hydromica into montmorillonite, with
formation of secondary montmorillonite.
DISCUSSION
According to the data cited above, a rather close relation exists between the various
clay-mineral contents and the thermobaric and hydrochemical characteristics of the
section in the South Caspian Basin and onshore of Azerbaijan. It is evident that all these
parameters interact, and the stability of montmorillonite at great depths depends mainly
on them. Moreover, in the section of Baku Archipelago at depths greater than 4 to 5 km,