Page 245 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 245
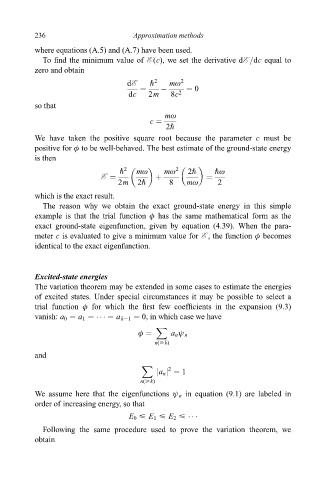
236 Approximation methods
where equations (A.5) and (A.7) have been used.
To ®nd the minimum value of E (c), we set the derivative dE =dc equal to
zero and obtain
dE " 2 mù 2
ÿ 0
dc 2m 8c 2
so that
mù
c
2"
We have taken the positive square root because the parameter c must be
positive for ö to be well-behaved. The best estimate of the ground-state energy
is then
" 2 mù mù 2 2" "ù
E
2m 2" 8 mù 2
which is the exact result.
The reason why we obtain the exact ground-state energy in this simple
example is that the trial function ö has the same mathematical form as the
exact ground-state eigenfunction, given by equation (4.39). When the para-
meter c is evaluated to give a minimum value for E , the function ö becomes
identical to the exact eigenfunction.
Excited-state energies
The variation theorem may be extended in some cases to estimate the energies
of excited states. Under special circumstances it may be possible to select a
trial function ö for which the ®rst few coef®cients in the expansion (9.3)
...
vanish: a 0 a 1 a kÿ1 0, in which case we have
X
ö a n ø n
n(>k)
and
X 2
ja n j 1
n(>k)
We assume here that the eigenfunctions ø n in equation (9.1) are labeled in
order of increasing energy, so that
E 0 < E 1 < E 2 <
Following the same procedure used to prove the variation theorem, we
obtain