Page 164 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 164
SORPTION FROM WATER SOLUTION 155
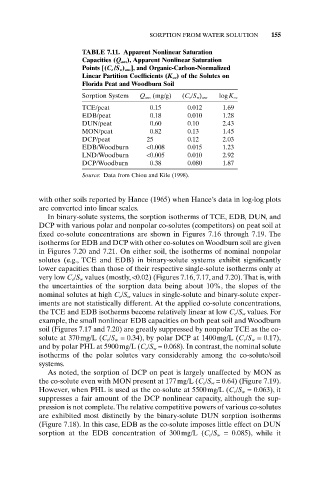
TABLE 7.11. Apparent Nonlinear Saturation
Capacities (Q ans), Apparent Nonlinear Saturation
Points [(C e /S w) ans], and Organic-Carbon-Normalized
Linear Partition Coefficients (K oc) of the Solutes on
Florida Peat and Woodburn Soil
Sorption System Q ans (mg/g) (C e/S w) ans logK oc
TCE/peat 0.15 0.012 1.69
EDB/peat 0.18 0.010 1.28
DUN/peat 0.60 0.10 2.43
MON/peat 0.82 0.13 1.45
DCP/peat 25 0.12 2.03
EDB/Woodburn <0.008 0.015 1.23
LND/Woodburn <0.005 0.010 2.92
DCP/Woodburn 0.38 0.080 1.87
Source: Data from Chiou and Kile (1998).
with other soils reported by Hance (1965) when Hance’s data in log-log plots
are converted into linear scales.
In binary-solute systems, the sorption isotherms of TCE, EDB, DUN, and
DCP with various polar and nonpolar co-solutes (competitors) on peat soil at
fixed co-solute concentrations are shown in Figures 7.16 through 7.19. The
isotherms for EDB and DCP with other co-solutes on Woodburn soil are given
in Figures 7.20 and 7.21. On either soil, the isotherms of nominal nonpolar
solutes (e.g., TCE and EDB) in binary-solute systems exhibit significantly
lower capacities than those of their respective single-solute isotherms only at
very low C e/S w values (mostly, <0.02) (Figures 7.16, 7.17, and 7.20). That is, with
the uncertainties of the sorption data being about 10%, the slopes of the
nominal solutes at high C e /S w values in single-solute and binary-solute exper-
iments are not statistically different. At the applied co-solute concentrations,
the TCE and EDB isotherms become relatively linear at low C e /S w values. For
example, the small nonlinear EDB capacities on both peat soil and Woodburn
soil (Figures 7.17 and 7.20) are greatly suppressed by nonpolar TCE as the co-
solute at 370mg/L (C e /S w = 0.34), by polar DCP at 1400mg/L (C e /S w = 0.17),
and by polar PHL at 5900mg/L (C e /S w = 0.068). In contrast, the nominal solute
isotherms of the polar solutes vary considerably among the co-solute/soil
systems.
As noted, the sorption of DCP on peat is largely unaffected by MON as
the co-solute even with MON present at 177mg/L (C e/S w = 0.64) (Figure 7.19).
However, when PHL is used as the co-solute at 5500mg/L (C e/S w = 0.063), it
suppresses a fair amount of the DCP nonlinear capacity, although the sup-
pression is not complete. The relative competitive powers of various co-solutes
are exhibited most distinctly by the binary-solute DUN sorption isotherms
(Figure 7.18). In this case, EDB as the co-solute imposes little effect on DUN
sorption at the EDB concentration of 300mg/L (C e/S w = 0.085), while it