Page 176 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 176
SORPTION FROM WATER SOLUTION 167
be attributed to the abundance of hydration-unaffected siloxane surfaces
available for atrazine adsorption.
Among the K-saturated clays, the adsorption powers observed follow the
order montmorillonite > illite > kaolinite, which reflects largely the amounts
of siloxane surfaces present in these clays. Here illite is less effective than
montmorillonite, presumably because the former has a much higher surface
charge (or charge density), making the surface more hydrophilic. An example
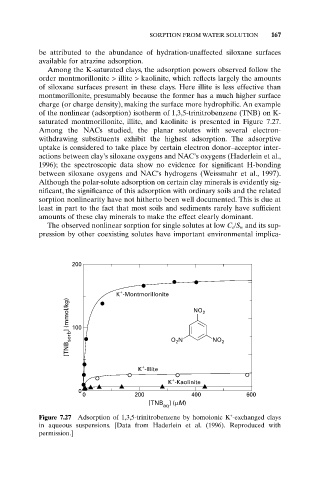
of the nonlinear (adsorption) isotherm of 1,3,5-trinitrobenzene (TNB) on K-
saturated montmorillonite, illite, and kaolinite is presented in Figure 7.27.
Among the NACs studied, the planar solutes with several electron-
withdrawing substituents exhibit the highest adsorption. The adsorptive
uptake is considered to take place by certain electron donor–acceptor inter-
actions between clay’s siloxane oxygens and NAC’s oxygens (Haderlein et al.,
1996); the spectroscopic data show no evidence for significant H-bonding
between siloxane oxygens and NAC’s hydrogens (Weissmahr et al., 1997).
Although the polar-solute adsorption on certain clay minerals is evidently sig-
nificant, the significance of this adsorption with ordinary soils and the related
sorption nonlinearity have not hitherto been well documented. This is due at
least in part to the fact that most soils and sediments rarely have sufficient
amounts of these clay minerals to make the effect clearly dominant.
The observed nonlinear sorption for single solutes at low C e/S w and its sup-
pression by other coexisting solutes have important environmental implica-
200
+
K -Montmorillonite NO
[TNB sorb ] (mmol/kg) 100 O N 2 NO 2
2
+
K -Illite
+
K -Kaolinite
0
0 200 400 600
[TNB ] (µM)
aq
+
Figure 7.27 Adsorption of 1,3,5-trinitrobenzene by homoionic K -exchanged clays
in aqueous suspensions. [Data from Haderlein et al. (1996). Reproduced with
permission.]