Page 198 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 198
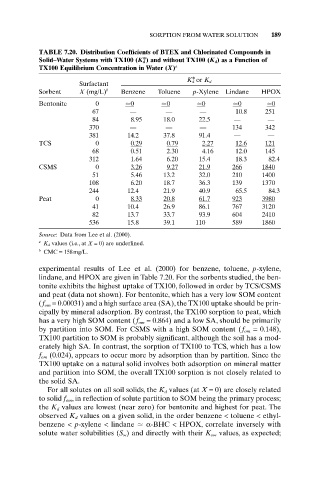
SORPTION FROM WATER SOLUTION 189
TABLE 7.20. Distribution Coefficients of BTEX and Chlorinated Compounds in
Solid–Water Systems with TX100 (K*) and without TX100 (K d) as a Function of
d
TX100 Equilibrium Concentration in Water (X) a
d
K* or K d
Surfactant
Sorbent X (mg/L) b Benzene Toluene p-Xylene Lindane HPOX
Bentonite 0 0 0 0 0 0
67 — — — 10.8 251
84 8.95 18.0 22.5 — —
370 — — — 134 342
381 14.2 37.8 91.4 — —
TCS 0 0.29 0.79 2.27 12.6 121
68 0.51 2.30 4.16 12.0 145
312 1.64 6.20 15.4 18.3 82.4
CSMS 0 3.26 9.27 21.9 266 1840
51 5.46 13.2 32.0 210 1400
108 6.20 18.7 36.3 139 1370
244 12.4 21.9 40.9 65.5 84.3
Peat 0 8.33 20.8 61.7 923 3980
41 10.4 26.9 86.1 767 3120
82 13.7 33.7 93.9 604 2410
536 15.8 39.1 110 589 1860
Source: Data from Lee et al. (2000).
a
K d values (i.e., at X = 0) are underlined.
b
CMC = 158mg/L.
experimental results of Lee et al. (2000) for benzene, toluene, p-xylene,
lindane, and HPOX are given in Table 7.20. For the sorbents studied, the ben-
tonite exhibits the highest uptake of TX100, followed in order by TCS/CSMS
and peat (data not shown). For bentonite, which has a very low SOM content
(f om = 0.00031) and a high surface area (SA), the TX100 uptake should be prin-
cipally by mineral adsorption. By contrast, the TX100 sorption to peat, which
has a very high SOM content (f om = 0.864) and a low SA, should be primarily
by partition into SOM. For CSMS with a high SOM content (f om = 0.148),
TX100 partition to SOM is probably significant, although the soil has a mod-
erately high SA. In contrast, the sorption of TX100 to TCS, which has a low
f om (0.024), appears to occur more by adsorption than by partition. Since the
TX100 uptake on a natural solid involves both adsorption on mineral matter
and partition into SOM, the overall TX100 sorption is not closely related to
the solid SA.
For all solutes on all soil solids, the K d values (at X = 0) are closely related
to solid f om, in reflection of solute partition to SOM being the primary process;
the K d values are lowest (near zero) for bentonite and highest for peat. The
observed K d values on a given solid, in the order benzene < toluene < ethyl-
benzene < p-xylene < lindane a-BHC < HPOX, correlate inversely with
solute water solubilities (S w) and directly with their K ow values, as expected;