Page 202 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 202
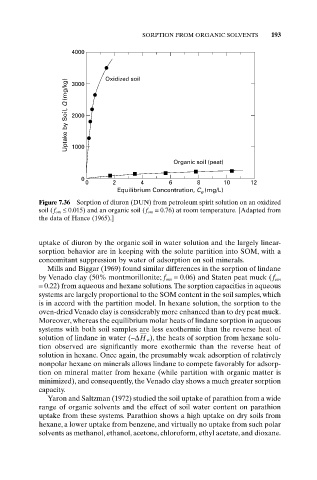
SORPTION FROM ORGANIC SOLVENTS 193
4000 Oxidized soil
Uptake by Soil, Q (mg/kg) 3000
2000
1000
Organic soil (peat)
0
0 2 4 6 8 10 12
Equilibrium Concentration, C (mg/L)
e
Figure 7.36 Sorption of diuron (DUN) from petroleum spirit solution on an oxidized
soil (f om £ 0.015) and an organic soil (f om = 0.76) at room temperature. [Adapted from
the data of Hance (1965).]
uptake of diuron by the organic soil in water solution and the largely linear-
sorption behavior are in keeping with the solute partition into SOM, with a
concomitant suppression by water of adsorption on soil minerals.
Mills and Biggar (1969) found similar differences in the sorption of lindane
by Venado clay (50% montmorillonite; f om = 0.06) and Staten peat muck (f om
= 0.22) from aqueous and hexane solutions. The sorption capacities in aqueous
systems are largely proportional to the SOM content in the soil samples, which
is in accord with the partition model. In hexane solution, the sorption to the
oven-dried Venado clay is considerably more enhanced than to dry peat muck.
Moreover, whereas the equilibrium molar heats of lindane sorption in aqueous
systems with both soil samples are less exothermic than the reverse heat of
solution of lindane in water (-DH w), the heats of sorption from hexane solu-
tion observed are significantly more exothermic than the reverse heat of
solution in hexane. Once again, the presumably weak adsorption of relatively
nonpolar hexane on minerals allows lindane to compete favorably for adsorp-
tion on mineral matter from hexane (while partition with organic matter is
minimized), and consequently, the Venado clay shows a much greater sorption
capacity.
Yaron and Saltzman (1972) studied the soil uptake of parathion from a wide
range of organic solvents and the effect of soil water content on parathion
uptake from these systems. Parathion shows a high uptake on dry soils from
hexane, a lower uptake from benzene, and virtually no uptake from such polar
solvents as methanol, ethanol, acetone, chloroform, ethyl acetate, and dioxane.