Page 204 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 204
SORPTION FROM ORGANIC SOLVENTS 195
5000
4000 Parathion
Uptake by Soil, Q (µg/g) 3000 2,4' -PCB
Lindane
1,2 -Dichlorobenzene
2000
1000
0
0 200 400 600
Equilibrium Concentration, C (mg/L)
e
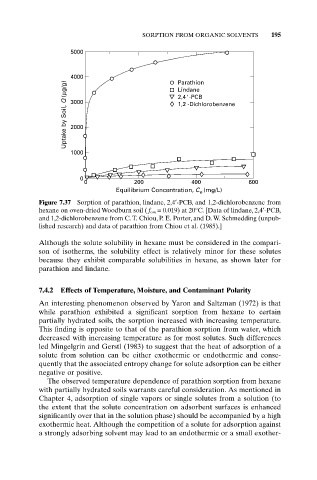
Figure 7.37 Sorption of parathion, lindane, 2,4¢-PCB, and 1,2-dichlorobenzene from
hexane on oven-dried Woodburn soil ( f om = 0.019) at 20°C. [Data of lindane, 2,4¢-PCB,
and 1,2-dichlorobenzene from C. T. Chiou, P. E. Porter, and D. W. Schmedding (unpub-
lished research) and data of parathion from Chiou et al. (1985).]
Although the solute solubility in hexane must be considered in the compari-
son of isotherms, the solubility effect is relatively minor for these solutes
because they exhibit comparable solubilities in hexane, as shown later for
parathion and lindane.
7.4.2 Effects of Temperature, Moisture, and Contaminant Polarity
An interesting phenomenon observed by Yaron and Saltzman (1972) is that
while parathion exhibited a significant sorption from hexane to certain
partially hydrated soils, the sorption increased with increasing temperature.
This finding is opposite to that of the parathion sorption from water, which
decreased with increasing temperature as for most solutes. Such differences
led Mingelgrin and Gerstl (1983) to suggest that the heat of adsorption of a
solute from solution can be either exothermic or endothermic and conse-
quently that the associated entropy change for solute adsorption can be either
negative or positive.
The observed temperature dependence of parathion sorption from hexane
with partially hydrated soils warrants careful consideration. As mentioned in
Chapter 4, adsorption of single vapors or single solutes from a solution (to
the extent that the solute concentration on adsorbent surfaces is enhanced
significantly over that in the solution phase) should be accompanied by a high
exothermic heat. Although the competition of a solute for adsorption against
a strongly adsorbing solvent may lead to an endothermic or a small exother-