Page 208 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 208
SORPTION FROM ORGANIC SOLVENTS 199
consistent with the lower polarity of lindane relative to parathion, making
lindane a less potent adsorbate, and thus a much weaker competitor than
parathion against water for mineral adsorption. Being a relatively nonpolar
adsorbate, lindane must exhibit a considerably smaller heat of adsorption per
unit area than water on dry soil minerals and consequently it cannot effec-
tively displace water from a partially hydrated soil. Thus, when all mineral sur-
faces are essentially covered by water, as for Woodburn soil with about 2.5%
water, the adsorption of lindane from hexane takes place presumably on less
energetic and more uniform adsorbed-water surfaces, resulting in a weak and
essentially linear uptake. This feature is in contrast to that found for parathion
under the same system condition.
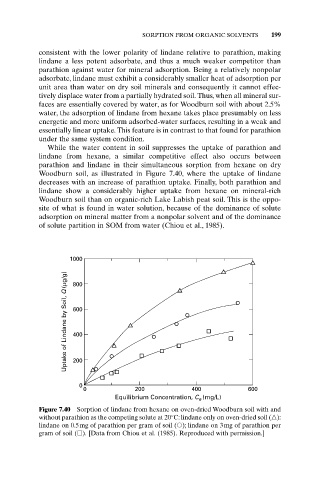
While the water content in soil suppresses the uptake of parathion and
lindane from hexane, a similar competitive effect also occurs between
parathion and lindane in their simultaneous sorption from hexane on dry
Woodburn soil, as illustrated in Figure 7.40, where the uptake of lindane
decreases with an increase of parathion uptake. Finally, both parathion and
lindane show a considerably higher uptake from hexane on mineral-rich
Woodburn soil than on organic-rich Lake Labish peat soil. This is the oppo-
site of what is found in water solution, because of the dominance of solute
adsorption on mineral matter from a nonpolar solvent and of the dominance
of solute partition in SOM from water (Chiou et al., 1985).
1000
Uptake of Lindane by Soil, Q (µg/g) 600
800
400
200
0
0 200 400 600
Equilibrium Concentration, C (mg/L)
e
Figure 7.40 Sorption of lindane from hexane on oven-dried Woodburn soil with and
without parathion as the competing solute at 20°C: lindane only on oven-dried soil ( ):
lindane on 0.5mg of parathion per gram of soil ( ); lindane on 3mg of parathion per
gram of soil ( ). [Data from Chiou et al. (1985). Reproduced with permission.]