Page 207 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 207
198 CONTAMINANT SORPTION TO SOILS AND NATURAL SOLIDS
The isosteric heat of parathion uptake determined from 20°C and 30°C
isotherms with dry Woodburn soil is highly exothermic (more than the reverse
heat of solution of parathion in hexane, -DH h) and varies with the parathion
loading, as shown in Figure 7.38b. These characteristics support the contention
that parathion adsorption on soil minerals is the dominant sorption mecha-
nism from a nonpolar solvent (hexane), as discussed previously. The high net
exothermic heat per mole of parathion adsorption (up to 70kJ/mol) comes
presumably from the simultaneous interactions of parathion’s many polar
groups with relatively polar mineral surfaces. Thus, on a per mole basis, the
heat of parathion adsorption can be greater than for water, whereas the heat
of adsorption per unit mineral surface must be greater for water to account
for the suppression of parathion adsorption by water.
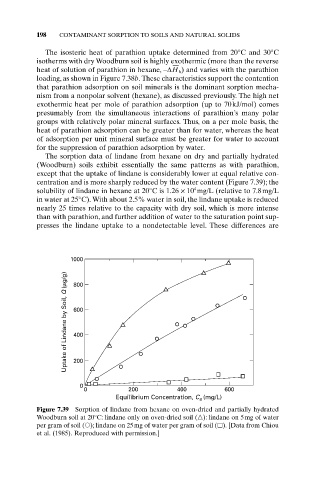
The sorption data of lindane from hexane on dry and partially hydrated
(Woodburn) soils exhibit essentially the same patterns as with parathion,
except that the uptake of lindane is considerably lower at equal relative con-
centration and is more sharply reduced by the water content (Figure 7.39); the
4
solubility of lindane in hexane at 20°C is 1.26 ¥ 10 mg/L (relative to 7.8mg/L
in water at 25°C). With about 2.5% water in soil, the lindane uptake is reduced
nearly 25 times relative to the capacity with dry soil, which is more intense
than with parathion, and further addition of water to the saturation point sup-
presses the lindane uptake to a nondetectable level. These differences are
1000
Uptake of Lindane by Soil, Q (µg/g) 600
800
400
200
0
0 200 400 600
Equilibrium Concentration, C (mg/L)
e
Figure 7.39 Sorption of lindane from hexane on oven-dried and partially hydrated
Woodburn soil at 20°C: lindane only on oven-dried soil ( ): lindane on 5mg of water
per gram of soil ( ); lindane on 25mg of water per gram of soil ( ). [Data from Chiou
et al. (1985). Reproduced with permission.]