Page 212 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 212
SORPTION FROM VAPOR PHASE 203
Partition Capacity, Q om (mg/g) 600
o 400
200
0
0 8 16 24
δ
Solubility Parameter, (cal/cm )
3 0.5
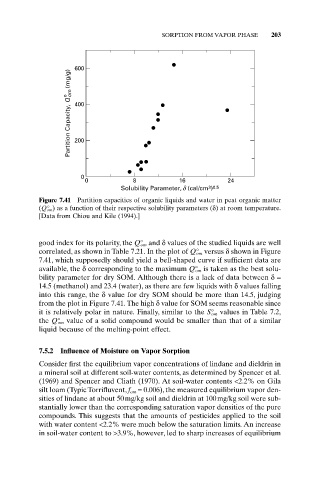
Figure 7.41 Partition capacities of organic liquids and water in peat organic matter
(Q° om ) as a function of their respective solubility parameters (d) at room temperature.
[Data from Chiou and Kile (1994).]
good index for its polarity, the Q° om and d values of the studied liquids are well
correlated, as shown in Table 7.21. In the plot of Q° om versus d shown in Figure
7.41, which supposedly should yield a bell-shaped curve if sufficient data are
available, the d corresponding to the maximum Q° om is taken as the best solu-
bility parameter for dry SOM. Although there is a lack of data between d=
14.5 (methanol) and 23.4 (water), as there are few liquids with d values falling
into this range, the d value for dry SOM should be more than 14.5, judging
from the plot in Figure 7.41. The high d value for SOM seems reasonable since
it is relatively polar in nature. Finally, similar to the S° om values in Table 7.2,
the Q° om value of a solid compound would be smaller than that of a similar
liquid because of the melting-point effect.
7.5.2 Influence of Moisture on Vapor Sorption
Consider first the equilibrium vapor concentrations of lindane and dieldrin in
a mineral soil at different soil-water contents, as determined by Spencer et al.
(1969) and Spencer and Cliath (1970). At soil-water contents <2.2% on Gila
silt loam (Typic Torrifluvent, f om = 0.006), the measured equilibrium vapor den-
sities of lindane at about 50mg/kg soil and dieldrin at 100mg/kg soil were sub-
stantially lower than the corresponding saturation vapor densities of the pure
compounds. This suggests that the amounts of pesticides applied to the soil
with water content <2.2% were much below the saturation limits. An increase
in soil-water content to >3.9%, however, led to sharp increases of equilibrium