Page 215 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 215
206 CONTAMINANT SORPTION TO SOILS AND NATURAL SOLIDS
40
Water (23.8°C)
1,2,4-Trichlorobenzene
Chlorobenzene
m-Dichlorobenzene
Benzene
30
p-Dichlorobenzene
Uptake by Soil, Q (mg/g) 20
10
0
0 0.2 0.4 0.6 0.8 1.0
Relative Pressure, P/P°
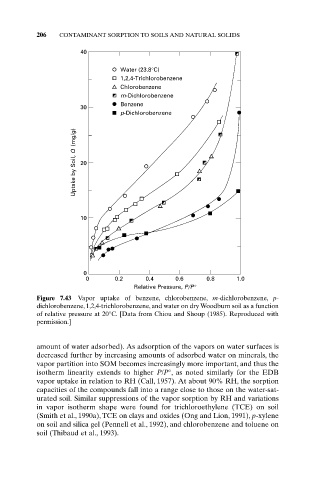
Figure 7.43 Vapor uptake of benzene, chlorobenzene, m-dichlorobenzene, p-
dichlorobenzene, 1,2,4-trichlorobenzene, and water on dry Woodburn soil as a function
of relative pressure at 20°C. [Data from Chiou and Shoup (1985). Reproduced with
permission.]
amount of water adsorbed). As adsorption of the vapors on water surfaces is
decreased further by increasing amounts of adsorbed water on minerals, the
vapor partition into SOM becomes increasingly more important, and thus the
isotherm linearity extends to higher P/P°, as noted similarly for the EDB
vapor uptake in relation to RH (Call, 1957). At about 90% RH, the sorption
capacities of the compounds fall into a range close to those on the water-sat-
urated soil. Similar suppressions of the vapor sorption by RH and variations
in vapor isotherm shape were found for trichloroethylene (TCE) on soil
(Smith et al., 1990a), TCE on clays and oxides (Ong and Lion, 1991), p-xylene
on soil and silica gel (Pennell et al., 1992), and chlorobenzene and toluene on
soil (Thibaud et al., 1993).