Page 219 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 219
210 CONTAMINANT SORPTION TO SOILS AND NATURAL SOLIDS
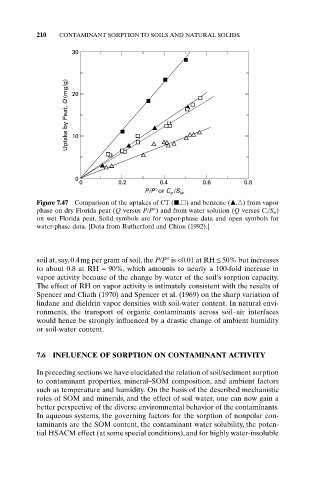
30
Uptake by Peat, Q (mg/g) 20
10
0
0 0.2 0.4 0.6 0.8
P/P°or C /S
e w
Figure 7.47 Comparison of the uptakes of CT ( , ) and benzene ( , ) from vapor
phase on dry Florida peat (Q versus P/P°) and from water solution (Q versus C e/S w)
on wet Florida peat. Solid symbols are for vapor-phase data and open symbols for
water-phase data. [Data from Rutherford and Chiou (1992).]
soil at, say, 0.4mg per gram of soil, the P/P° is <0.01 at RH £ 50% but increases
to about 0.8 at RH = 90%, which amounts to nearly a 100-fold increase in
vapor activity because of the change by water of the soil’s sorption capacity.
The effect of RH on vapor activity is intimately consistent with the results of
Spencer and Cliath (1970) and Spencer et al. (1969) on the sharp variation of
lindane and dieldrin vapor densities with soil-water content. In natural envi-
ronments, the transport of organic contaminants across soil–air interfaces
would hence be strongly influenced by a drastic change of ambient humidity
or soil-water content.
7.6 INFLUENCE OF SORPTION ON CONTAMINANT ACTIVITY
In preceding sections we have elucidated the relation of soil/sediment sorption
to contaminant properties, mineral–SOM composition, and ambient factors
such as temperature and humidity. On the basis of the described mechanistic
roles of SOM and minerals, and the effect of soil water, one can now gain a
better perspective of the diverse environmental behavior of the contaminants.
In aqueous systems, the governing factors for the sorption of nonpolar con-
taminants are the SOM content, the contaminant water solubility, the poten-
tial HSACM effect (at some special conditions), and for highly water-insoluble