Page 216 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 216
SORPTION FROM VAPOR PHASE 207
40
0% R.H.
30
Uptake by Soil, Q (mg/g) 20
18% R.H.
50% R.H.
10
90% R.H.
0
0 0.2 0.4 0.6 0.8 1.0
Relative Pressure, P/P°
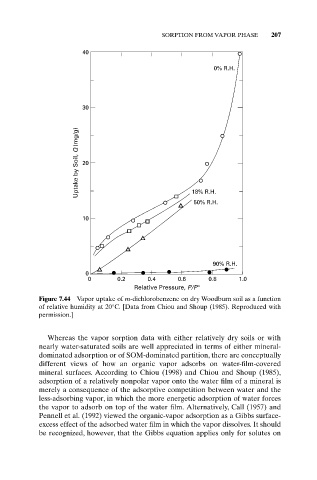
Figure 7.44 Vapor uptake of m-dichlorobenzene on dry Woodburn soil as a function
of relative humidity at 20°C. [Data from Chiou and Shoup (1985). Reproduced with
permission.]
Whereas the vapor sorption data with either relatively dry soils or with
nearly water-saturated soils are well appreciated in terms of either mineral-
dominated adsorption or of SOM-dominated partition, there are conceptually
different views of how an organic vapor adsorbs on water-film-covered
mineral surfaces. According to Chiou (1998) and Chiou and Shoup (1985),
adsorption of a relatively nonpolar vapor onto the water film of a mineral is
merely a consequence of the adsorptive competition between water and the
less-adsorbing vapor, in which the more energetic adsorption of water forces
the vapor to adsorb on top of the water film. Alternatively, Call (1957) and
Pennell et al. (1992) viewed the organic-vapor adsorption as a Gibbs surface-
excess effect of the adsorbed water film in which the vapor dissolves. It should
be recognized, however, that the Gibbs equation applies only for solutes on