Page 217 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 217
208 CONTAMINANT SORPTION TO SOILS AND NATURAL SOLIDS
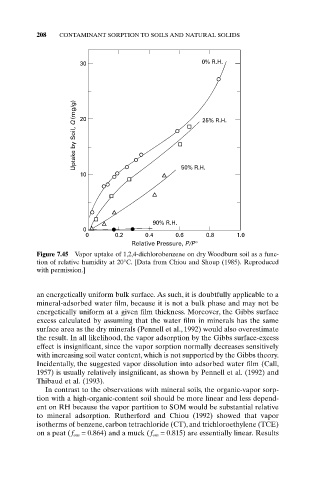
30 0% R.H.
Uptake by Soil, Q (mg/g) 20 25% R.H.
10 50% R.H.
90% R.H.
0
0 0.2 0.4 0.6 0.8 1.0
Relative Pressure, P/P°
Figure 7.45 Vapor uptake of 1,2,4-dichlorobenzene on dry Woodburn soil as a func-
tion of relative humidity at 20°C. [Data from Chiou and Shoup (1985). Reproduced
with permission.]
an energetically uniform bulk surface. As such, it is doubtfully applicable to a
mineral-adsorbed water film, because it is not a bulk phase and may not be
energetically uniform at a given film thickness. Moreover, the Gibbs surface
excess calculated by assuming that the water film in minerals has the same
surface area as the dry minerals (Pennell et al., 1992) would also overestimate
the result. In all likelihood, the vapor adsorption by the Gibbs surface-excess
effect is insignificant, since the vapor sorption normally decreases sensitively
with increasing soil water content, which is not supported by the Gibbs theory.
Incidentally, the suggested vapor dissolution into adsorbed water film (Call,
1957) is usually relatively insignificant, as shown by Pennell et al. (1992) and
Thibaud et al. (1993).
In contrast to the observations with mineral soils, the organic-vapor sorp-
tion with a high-organic-content soil should be more linear and less depend-
ent on RH because the vapor partition to SOM would be substantial relative
to mineral adsorption. Rutherford and Chiou (1992) showed that vapor
isotherms of benzene, carbon tetrachloride (CT), and trichloroethylene (TCE)
on a peat (f om = 0.864) and a muck (f om = 0.815) are essentially linear. Results