Page 196 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 196
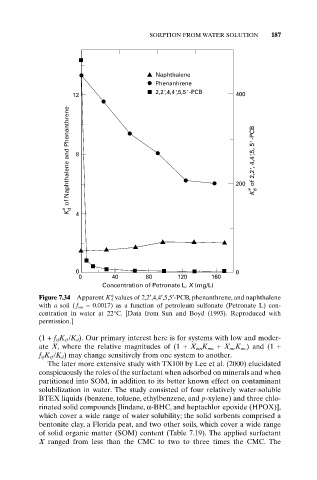
SORPTION FROM WATER SOLUTION 187
Naphthalene
Phenanhrene
2,2',4,4',5,5' -PCB
12 400
K d of Naphthalene and Phenanthrene 8 200 K d of 2,2', 4,4',5, 5' -PCB
* 4 *
0 0
0 40 80 120 160
Concentration of Petronate L, X (mg/L)
Figure 7.34 Apparent K* values of 2,2¢,4,4¢,5,5¢-PCB, phenanthrene, and naphthalene
d
with a soil ( f om = 0.0017) as a function of petroleum sulfonate (Petronate L) con-
centration in water at 22°C. [Data from Sun and Boyd (1993). Reproduced with
permission.]
(1 + f sf K sf /K d ). Our primary interest here is for systems with low and moder-
ate X, where the relative magnitudes of (1 + X mn K mn + X mc K mc ) and (1 +
f sf K sf /K d ) may change sensitively from one system to another.
The later more extensive study with TX100 by Lee et al. (2000) elucidated
conspicuously the roles of the surfactant when adsorbed on minerals and when
partitioned into SOM, in addition to its better known effect on contaminant
solubilization in water. The study consisted of four relatively water-soluble
BTEX liquids (benzene, toluene, ethylbenzene, and p-xylene) and three chlo-
rinated solid compounds [lindane, a-BHC, and heptachlor epoxide (HPOX)],
which cover a wide range of water solubility; the solid sorbents comprised a
bentonite clay, a Florida peat, and two other soils, which cover a wide range
of solid organic matter (SOM) content (Table 7.19). The applied surfactant
X ranged from less than the CMC to two to three times the CMC. The