Page 288 - Petroleum and Gas Field Processing
P. 288
when the CO 2 partial pressure is in the range 30–90 psi. The following
reactions occur in this case:
K 2 CO 3 þ CO 2 þ H 2 O , 2KHCO 3 ð12Þ
K 2 CO 3 þ H 2 S , KHS þ KHCO 3 ð13Þ
It can be seen from reaction (12) that a high partial pressure of CO 2 is
required to keep KHCO 3 in solution, and in Eq. (13), H 2 S will not react if
the CO 2 pressure is not high. For this reason, this process cannot achieve a
low concentration of acid gases in the exit stream and a polishing process
is needed (molecular sieve). An elevated temperature is also necessary to
ensure that potassium carbonate and reaction products (KHCO 3 and
KHS) remain in solution. Thus, this process cannot be used for gases
containing H 2 S only.
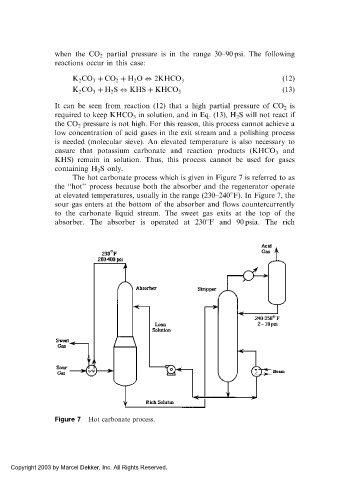
The hot carbonate process which is given in Figure 7 is referred to as
the ‘‘hot’’ process because both the absorber and the regenerator operate
at elevated temperatures, usually in the range (230–240 F). In Figure 7, the
sour gas enters at the bottom of the absorber and flows countercurrently
to the carbonate liquid stream. The sweet gas exits at the top of the
absorber. The absorber is operated at 230 F and 90 psia. The rich
Figure 7 Hot carbonate process.
Copyright 2003 by Marcel Dekker, Inc. All Rights Reserved.