Page 285 - Petrophysics 2E
P. 285
258 PETROPHYSICS: RESERVOIR ROCK PROPERTIES
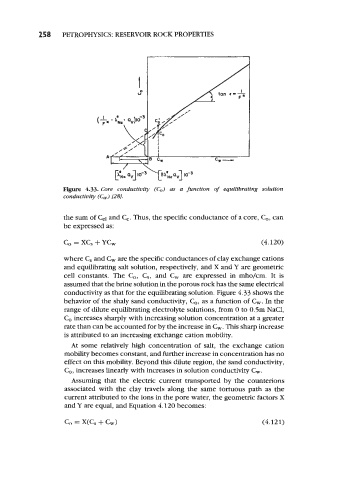
Figure 4.33. Core conductivity (C,) as a function of equilibrating solution
conductivity (C,) [28J
the sum of C,l and &. Thus, the specific conductance of a core, C,, can
be expressed as:
co = xcs + YC, (4.120)
where C, and C, are the specific conductances of clay exchange cations
and equilibrating salt solution, respectively, and X and Y are geometric
cell constants. The Co, Cs, and C, are expressed in mho/cm. It is
assumed that the brine solution in the porous rock has the same electrical
conductivity as that for the equilibrating solution. Figure 4.33 shows the
behavior of the shaly sand conductivity, C,, as a function of &. In the
range of dilute equilibrating electrolyte solutions, from 0 to 0.5m NaCl,
Co increases sharply with increasing solution concentration at a greater
rate than can be accounted for by the increase in C&. This sharp increase
is attributed to an increasing exchange cation mobility.
At some relatively high concentration of salt, the exchange cation
mobility becomes constant, and further increase in concentration has no
effect on this mobility. Beyond this dilute region, the sand conductivity,
C,, increases linearly with increases in solution conductivity C&.
Assuming that the electric current transported by the counterions
associated with the clay travels along the same tortuous path as the
current attributed to the ions in the pore water, the geometric factors X
and Y are equal, and Equation 4.120 becomes: