Page 87 - Photoreactive Organic Thin Films
P. 87
66 ZOUHEIR SEKKAT
azobenzene derivatives, a necessary groundwork for photo-orientation studies.
Azobenzene derivatives and other photoisomerizable molecules have two
geometric isomers, the trans and the cis forms, and the isomerization reaction
is a light- or heat-induced interconversion of the two isomers. (See the top
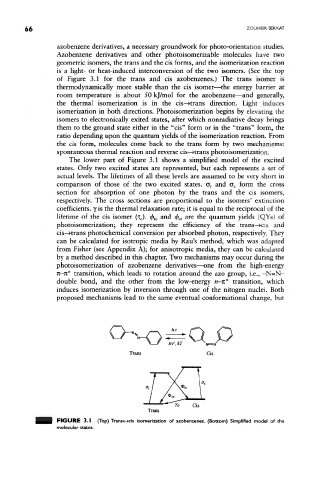
of Figure 3.1 for the trans and cis azobenzenes.) The trans isomer is
thermodynamically more stable than the cis isomer—the energy barrier at
room temperature is about 50 kj/mol for the azobenzene—and generally,
the thermal isomerization is in the cis—»trans direction. Light induces
isomerization in both directions. Photoisomerization begins by elevating the
isomers to electronically exited states, after which nonradiative decay brings
them to the ground state either in the "cis" form or in the "trans" form, the
ratio depending upon the quantum yields of the isomerization reaction. From
the cis form, molecules come back to the trans form by two mechanisms:
spontaneous thermal reaction and reverse cis—Hrans photoisomerization,.
The lower part of Figure 3.1 shows a simplified model of the excited
states. Only two excited states are represented, but each represents a set of
actual levels. The lifetimes of all these levels are assumed to be very short in
comparison of those of the two excited states. a, and <5 C form the cross
section for absorption of one photon by the trans and the cis isomers,
respectively. The cross sections are proportional to the isomers' extinction
coefficients, y is the thermal relaxation rate; it is equal to the reciprocal of the
lifetime of the cis isomer (i c). <f> tc and <$># are the quantum yields (QYs) of
photoisomerization; they represent the efficiency of the trans~»cis and
cis—>trans photochemical conversion per absorbed photon, respectively. They
can be calculated for isotropic media by Rau's method, which was adapted
from Fisher {see Appendix A); for anisotropic media, they can be calculated
by a method described in this chapter. Two mechanisms may occur during the
photoisomerization of azobenzene derivatives—one from the high-energy
n-n* transition, which leads to rotation around the azo group, i.e., -N=N-
double bond, and the other from the low-energy n-n* transition, which
induces isomerization by inversion through one of the nitogen nuclei. Both
proposed mechanisms lead to the same eventual conformational change, but
Yo Cis
Trans
FIGURE 3.1 (Top) Trans«-»cis isomerization of azobenzenes. (Bottom) Simplified model of the
molecular states.