Page 226 - Physical chemistry understanding our chemical world
P. 226
QUANTITATIVE EFFECTS OF PRESSURE AND TEMPERATURE CHANGE 193
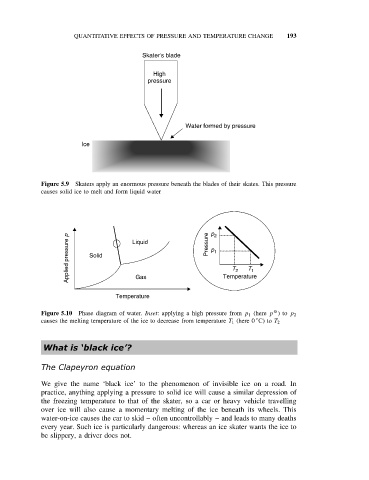
Skater's blade
High
pressure
Water formed by pressure
Ice
Figure 5.9 Skaters apply an enormous pressure beneath the blades of their skates. This pressure
causes solid ice to melt and form liquid water
Applied pressure p Solid Liquid Pressure p 2 1 Temperature
p
T
T
1
2
Gas
Temperature
O
Figure 5.10 Phase diagram of water. Inset: applying a high pressure from p 1 (here p )to p 2
◦
causes the melting temperature of the ice to decrease from temperature T 1 (here 0 C) to T 2
What is ‘black ice’?
The Clapeyron equation
We give the name ‘black ice’ to the phenomenon of invisible ice on a road. In
practice, anything applying a pressure to solid ice will cause a similar depression of
the freezing temperature to that of the skater, so a car or heavy vehicle travelling
over ice will also cause a momentary melting of the ice beneath its wheels. This
water-on-ice causes the car to skid – often uncontrollably – and leads to many deaths
every year. Such ice is particularly dangerous: whereas an ice skater wants the ice to
be slippery, a driver does not.