Page 222 - Physical chemistry understanding our chemical world
P. 222
PRESSURE AND TEMPERATURE CHANGES WITH A SINGLE-COMPONENT SYSTEM 189
How is coffee decaffeinated?
Critical and supercritical fluids
We continue our theme of ‘coffee’. Most coffees contain a large amount of the
heterocyclic stimulant caffeine (I). Some people prefer to decrease the amounts of
caffeine they ingest for health reasons, or they simply do not like to consume it at
all, and they ask for decaffeinated coffee instead.
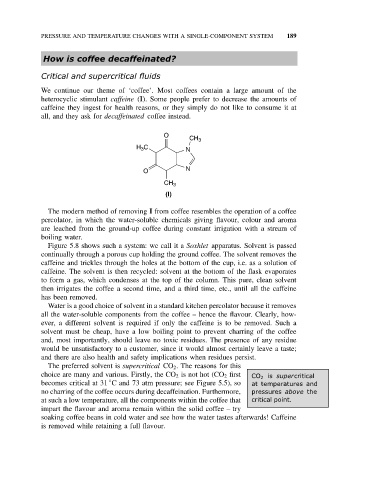
O
CH 3
H 3 C N
N
O
CH 3
(I)
The modern method of removing I from coffee resembles the operation of a coffee
percolator, in which the water-soluble chemicals giving flavour, colour and aroma
are leached from the ground-up coffee during constant irrigation with a stream of
boiling water.
Figure 5.8 shows such a system: we call it a Soxhlet apparatus. Solvent is passed
continually through a porous cup holding the ground coffee. The solvent removes the
caffeine and trickles through the holes at the bottom of the cup, i.e. as a solution of
caffeine. The solvent is then recycled: solvent at the bottom of the flask evaporates
to form a gas, which condenses at the top of the column. This pure, clean solvent
then irrigates the coffee a second time, and a third time, etc., until all the caffeine
has been removed.
Water is a good choice of solvent in a standard kitchen percolator because it removes
all the water-soluble components from the coffee – hence the flavour. Clearly, how-
ever, a different solvent is required if only the caffeine is to be removed. Such a
solvent must be cheap, have a low boiling point to prevent charring of the coffee
and, most importantly, should leave no toxic residues. The presence of any residue
would be unsatisfactory to a customer, since it would almost certainly leave a taste;
and there are also health and safety implications when residues persist.
The preferred solvent is supercritical CO 2 . The reasons for this
choice are many and various. Firstly, the CO 2 is not hot (CO 2 first CO 2 is supercritical
◦
becomes critical at 31 C and 73 atm pressure; see Figure 5.5), so at temperatures and
no charring of the coffee occurs during decaffeination. Furthermore, pressures above the
at such a low temperature, all the components within the coffee that critical point.
impart the flavour and aroma remain within the solid coffee – try
soaking coffee beans in cold water and see how the water tastes afterwards! Caffeine
is removed while retaining a full flavour.