Page 218 - Physical chemistry understanding our chemical world
P. 218
PRESSURE AND TEMPERATURE CHANGES WITH A SINGLE-COMPONENT SYSTEM 185
73
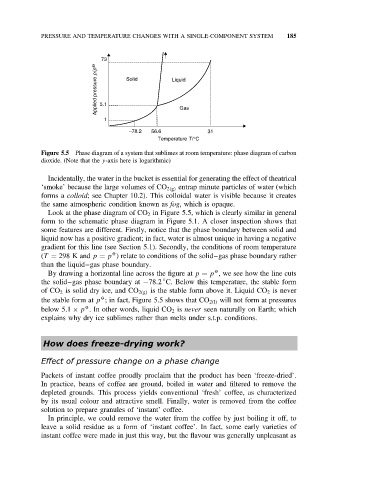
Applied pressure p/p O 5.1 Solid Liquid
1 Gas
−78.2 56.6 31
Temperature T/°C
Figure 5.5 Phase diagram of a system that sublimes at room temperature: phase diagram of carbon
dioxide. (Note that the y-axis here is logarithmic)
Incidentally, the water in the bucket is essential for generating the effect of theatrical
‘smoke’ because the large volumes of CO 2(g) entrap minute particles of water (which
forms a colloid; see Chapter 10.2). This colloidal water is visible because it creates
the same atmospheric condition known as fog, which is opaque.
Look at the phase diagram of CO 2 in Figure 5.5, which is clearly similar in general
form to the schematic phase diagram in Figure 5.1. A closer inspection shows that
some features are different. Firstly, notice that the phase boundary between solid and
liquid now has a positive gradient; in fact, water is almost unique in having a negative
gradient for this line (see Section 5.1). Secondly, the conditions of room temperature
O
(T = 298 K and p = p ) relate to conditions of the solid–gas phase boundary rather
than the liquid–gas phase boundary.
By drawing a horizontal line across the figure at p = p , we see how the line cuts
O
◦
the solid–gas phase boundary at −78.2 C. Below this temperature, the stable form
of CO 2 is solid dry ice, and CO 2(g) is the stable form above it. Liquid CO 2 is never
O
the stable form at p ; in fact, Figure 5.5 shows that CO 2(l) will not form at pressures
O
below 5.1 × p . In other words, liquid CO 2 is never seen naturally on Earth; which
explains why dry ice sublimes rather than melts under s.t.p. conditions.
How does freeze-drying work?
Effect of pressure change on a phase change
Packets of instant coffee proudly proclaim that the product has been ‘freeze-dried’.
In practice, beans of coffee are ground, boiled in water and filtered to remove the
depleted grounds. This process yields conventional ‘fresh’ coffee, as characterized
by its usual colour and attractive smell. Finally, water is removed from the coffee
solution to prepare granules of ‘instant’ coffee.
In principle, we could remove the water from the coffee by just boiling it off, to
leave a solid residue as a form of ‘instant coffee’. In fact, some early varieties of
instant coffee were made in just this way, but the flavour was generally unpleasant as