Page 223 - Physical chemistry understanding our chemical world
P. 223
190 PHASE EQUILIBRIA
Water out
Condenser
Cool, pure
fluid Water in Hot, pure
fluid
Coffee
beans to be
decaffeinated
Fluid
containing
caffeine
Reservoir, where
extracted
caffeine accumulates
Heat
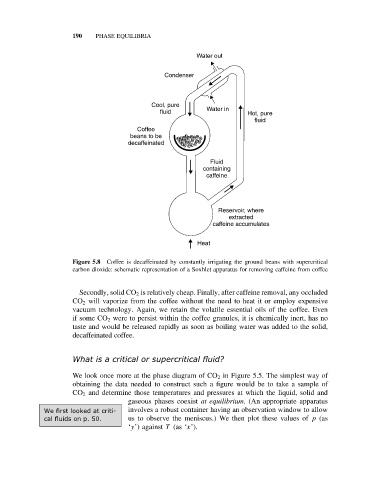
Figure 5.8 Coffee is decaffeinated by constantly irrigating the ground beans with supercritical
carbon dioxide: schematic representation of a Soxhlet apparatus for removing caffeine from coffee
Secondly, solid CO 2 is relatively cheap. Finally, after caffeine removal, any occluded
CO 2 will vaporize from the coffee without the need to heat it or employ expensive
vacuum technology. Again, we retain the volatile essential oils of the coffee. Even
if some CO 2 were to persist within the coffee granules, it is chemically inert, has no
taste and would be released rapidly as soon as boiling water was added to the solid,
decaffeinated coffee.
What is a critical or supercritical fluid?
We look once more at the phase diagram of CO 2 in Figure 5.5. The simplest way of
obtaining the data needed to construct such a figure would be to take a sample of
CO 2 and determine those temperatures and pressures at which the liquid, solid and
gaseous phases coexist at equilibrium. (An appropriate apparatus
We first looked at criti- involves a robust container having an observation window to allow
cal fluids on p. 50. us to observe the meniscus.) We then plot these values of p (as
‘y’) against T (as ‘x’).