Page 224 - Physical chemistry understanding our chemical world
P. 224
PRESSURE AND TEMPERATURE CHANGES WITH A SINGLE-COMPONENT SYSTEM 191
Let us consider more closely what happens as the conditions become more extreme
inside the observation can. As heating proceeds, so the amount of CO 2(l) convert-
ing to form gas increases. Accordingly, the amount of CO 2 within the gaseous
phase increases, which will cause the density ρ of the vapour to increase. Con-
versely, if we consider the liquid, at no time does its density alter
appreciably, even though its volume decreases as a result of liquid
It is impossible to dis-
forming vapour. tinguish between the
From a consideration of the relative densities, we expect the liquid and gaseous
liquid phase to reside at the bottom of the container, with the phases of CO 2 at tem-
less-dense gaseous phase ‘floating’ above it. The ‘critical’ point is peratures and pres-
reached when the density of the gas has increased until it becomes sures at and above the
the same as that of the liquid. In consequence, there is now no critical point.
longer a lighter and a heavier phase, because ρ (liquid) = ρ (vapour) .
Accordingly, we no longer see a meniscus separating liquid at the The intensive prop-
bottom of the container and vapour above it: it is impossible to see erties of the liquid
a clear distinction between the liquid and gas components. We say and gas (density, heat
that the CO 2 is critical. capacity, etc.) become
Further heating or additional increases in pressure generate equal at the criti-
supercritical CO 2 . The pressure and temperature at which the fluid cal point,which is
first becomes critical are respectively termed T (critical) and p (critical) . the highest temper-
ature and pressure at
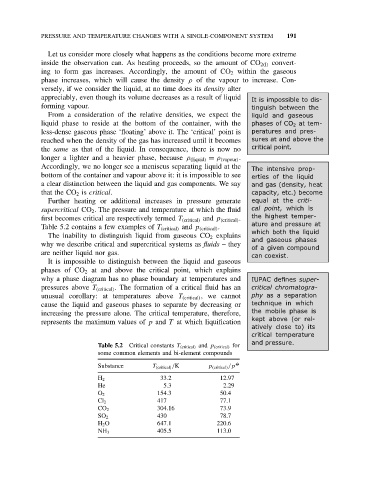
Table 5.2 contains a few examples of T (critical) and p (critical) .
which both the liquid
The inability to distinguish liquid from gaseous CO 2 explains
and gaseous phases
why we describe critical and supercritical systems as fluids – they of a given compound
are neither liquid nor gas.
can coexist.
It is impossible to distinguish between the liquid and gaseous
phases of CO 2 at and above the critical point, which explains
why a phase diagram has no phase boundary at temperatures and IUPAC defines super-
pressures above T (critical) . The formation of a critical fluid has an critical chromatogra-
unusual corollary: at temperatures above T (critical) , we cannot phy as a separation
cause the liquid and gaseous phases to separate by decreasing or technique in which
increasing the pressure alone. The critical temperature, therefore, themobile phaseis
kept above (or rel-
represents the maximum values of p and T at which liquification
atively close to) its
critical temperature
and pressure.
Table 5.2 Critical constants T (critical) and p (critical) for
some common elements and bi-element compounds
Substance T (critical) /K p (critical) /p O
H 2 33.2 12.97
He 5.3 2.29
O 2 154.3 50.4
Cl 2 417 77.1
CO 2 304.16 73.9
SO 2 430 78.7
H 2 O 647.1 220.6
NH 3 405.5 113.0