Page 219 - Physical chemistry understanding our chemical world
P. 219
186 PHASE EQUILIBRIA
a result of charring during prolonged heating. Clearly, a better method of removing
the water was required.
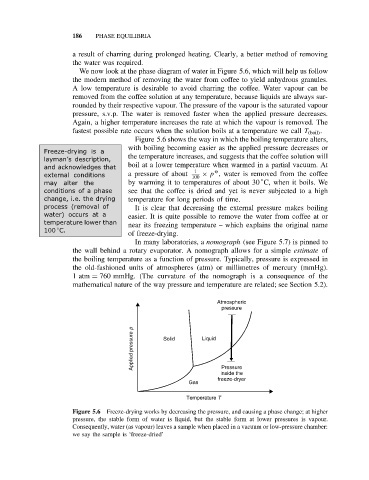
We now look at the phase diagram of water in Figure 5.6, which will help us follow
the modern method of removing the water from coffee to yield anhydrous granules.
A low temperature is desirable to avoid charring the coffee. Water vapour can be
removed from the coffee solution at any temperature, because liquids are always sur-
rounded by their respective vapour. The pressure of the vapour is the saturated vapour
pressure, s.v.p. The water is removed faster when the applied pressure decreases.
Again, a higher temperature increases the rate at which the vapour is removed. The
fastest possible rate occurs when the solution boils at a temperature we call T (boil) .
Figure 5.6 shows the way in which the boiling temperature alters,
with boiling becoming easier as the applied pressure decreases or
Freeze-drying is a
layman’s description, the temperature increases, and suggests that the coffee solution will
and acknowledges that boil at a lower temperature when warmed in a partial vacuum. At
1
O
external conditions a pressure of about 100 × p , water is removed from the coffee
◦
may alter the by warming it to temperatures of about 30 C, when it boils. We
conditions of a phase see that the coffee is dried and yet is never subjected to a high
change, i.e. the drying temperature for long periods of time.
process (removal of It is clear that decreasing the external pressure makes boiling
water) occurs at a easier. It is quite possible to remove the water from coffee at or
temperature lower than near its freezing temperature – which explains the original name
100 C.
◦
of freeze-drying.
In many laboratories, a nomograph (see Figure 5.7) is pinned to
the wall behind a rotary evaporator. A nomograph allows for a simple estimate of
the boiling temperature as a function of pressure. Typically, pressure is expressed in
the old-fashioned units of atmospheres (atm) or millimetres of mercury (mmHg).
1atm = 760 mmHg. (The curvature of the nomograph is a consequence of the
mathematical nature of the way pressure and temperature are related; see Section 5.2).
Atmospheric
pressure
Applied pressure p Solid Liquid
Pressure
inside the
freeze-dryer
Gas
Temperature T
Figure 5.6 Freeze-drying works by decreasing the pressure, and causing a phase change; at higher
pressure, the stable form of water is liquid, but the stable form at lower pressures is vapour.
Consequently, water (as vapour) leaves a sample when placed in a vacuum or low-pressure chamber:
we say the sample is ‘freeze-dried’