Page 308 - Physical chemistry understanding our chemical world
P. 308
ACID–BASE INDICATORS 275
OH OH
OH O
+
HO O HO O
OH O −
OH OH
OH OH
Red Blue
OH
O
HO O
OH
OH
OH
Mauve
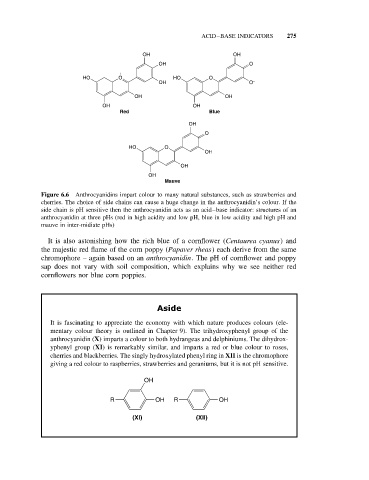
Figure 6.6 Anthrocyanidins impart colour to many natural substances, such as strawberries and
cherries. The choice of side chains can cause a huge change in the anthrocyanidin’s colour. If the
side chain is pH sensitive then the anthrocyanidin acts as an acid–base indicator: structures of an
anthrocyanidin at three pHs (red in high acidity and low pH, blue in low acidity and high pH and
mauve in inter-midiate pHs)
It is also astonishing how the rich blue of a cornflower (Centaurea cyanus) and
the majestic red flame of the corn poppy (Papaver rheas) each derive from the same
chromophore – again based on an anthrocyanidin. The pH of cornflower and poppy
sap does not vary with soil composition, which explains why we see neither red
cornflowers nor blue corn poppies.
Aside
It is fascinating to appreciate the economy with which nature produces colours (ele-
mentary colour theory is outlined in Chapter 9). The trihydroxyphenyl group of the
anthrocyanidin (X) imparts a colour to both hydrangeas and delphiniums. The dihydrox-
yphenyl group (XI) is remarkably similar, and imparts a red or blue colour to roses,
cherries and blackberries. The singly hydroxylated phenyl ring in XII is the chromophore
giving a red colour to raspberries, strawberries and geraniums, but it is not pH sensitive.
OH
R OH R OH
(XI) (XII)