Page 309 - Physical chemistry understanding our chemical world
P. 309
276 ACIDS AND BASES
Why does phenolphthalein indicator not turn red until
pH 8.2?
Which acid–base indicator to use?
Litmus was probably the most popular choice of acid–base indicator, but it is not a
good choice for colour-blind chemists. The use of phenolphthalein as an acid–base
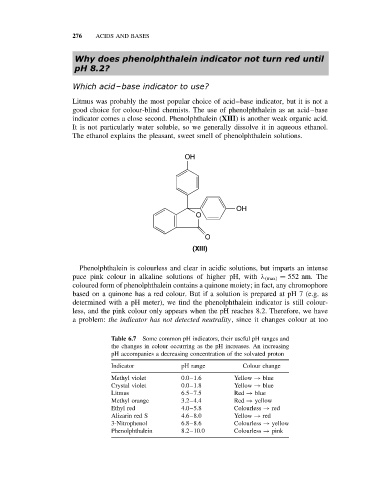
indicator comes a close second. Phenolphthalein (XIII) is another weak organic acid.
It is not particularly water soluble, so we generally dissolve it in aqueous ethanol.
The ethanol explains the pleasant, sweet smell of phenolphthalein solutions.
OH
OH
O
O
(XIII)
Phenolphthalein is colourless and clear in acidic solutions, but imparts an intense
puce pink colour in alkaline solutions of higher pH, with λ (max) = 552 nm. The
coloured form of phenolphthalein contains a quinone moiety; in fact, any chromophore
based on a quinone has a red colour. But if a solution is prepared at pH 7 (e.g. as
determined with a pH meter), we find the phenolphthalein indicator is still colour-
less, and the pink colour only appears when the pH reaches 8.2. Therefore, we have
a problem: the indicator has not detected neutrality, since it changes colour at too
Table 6.7 Some common pH indicators, their useful pH ranges and
the changes in colour occurring as the pH increases. An increasing
pH accompanies a decreasing concentration of the solvated proton
Indicator pH range Colour change
Methyl violet 0.0–1.6 Yellow → blue
Crystal violet 0.0–1.8 Yellow → blue
Litmus 6.5–7.5 Red → blue
Methyl orange 3.2–4.4 Red → yellow
Ethyl red 4.0–5.8 Colourless → red
Alizarin red S 4.6–8.0 Yellow → red
3-Nitrophenol 6.8–8.6 Colourless → yellow
Phenolphthalein 8.2–10.0 Colourless → pink