Page 339 - Physical chemistry understanding our chemical world
P. 339
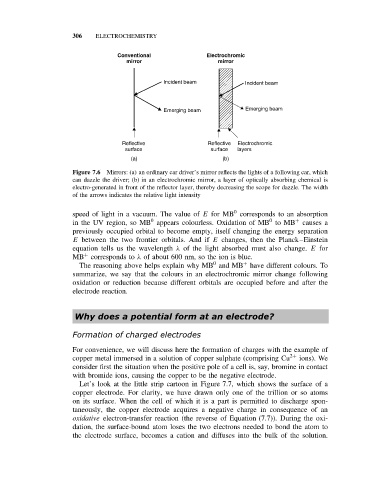
306 ELECTROCHEMISTRY
Conventional Electrochromic
mirror mirror
Incident beam Incident beam
Emerging beam Emerging beam
Reflective Reflective Electrochromic
surface surface layers
(a) (b)
Figure 7.6 Mirrors: (a) an ordinary car driver’s mirror reflects the lights of a following car, which
can dazzle the driver; (b) in an electrochromic mirror, a layer of optically absorbing chemical is
electro-generated in front of the reflector layer, thereby decreasing the scope for dazzle. The width
of the arrows indicates the relative light intensity
0
speed of light in a vacuum. The value of E for MB corresponds to an absorption
0
0
+
in the UV region, so MB appears colourless. Oxidation of MB to MB causes a
previously occupied orbital to become empty, itself changing the energy separation
E between the two frontier orbitals. And if E changes, then the Planck–Einstein
equation tells us the wavelength λ of the light absorbed must also change. E for
+
MB corresponds to λ of about 600 nm, so the ion is blue.
0
The reasoning above helps explain why MB and MB have different colours. To
+
summarize, we say that the colours in an electrochromic mirror change following
oxidation or reduction because different orbitals are occupied before and after the
electrode reaction.
Why does a potential form at an electrode?
Formation of charged electrodes
For convenience, we will discuss here the formation of charges with the example of
copper metal immersed in a solution of copper sulphate (comprising Cu 2+ ions). We
consider first the situation when the positive pole of a cell is, say, bromine in contact
with bromide ions, causing the copper to be the negative electrode.
Let’s look at the little strip cartoon in Figure 7.7, which shows the surface of a
copper electrode. For clarity, we have drawn only one of the trillion or so atoms
on its surface. When the cell of which it is a part is permitted to discharge spon-
taneously, the copper electrode acquires a negative charge in consequence of an
oxidative electron-transfer reaction (the reverse of Equation (7.7)). During the oxi-
dation, the surface-bound atom loses the two electrons needed to bond the atom to
the electrode surface, becomes a cation and diffuses into the bulk of the solution.