Page 340 - Physical chemistry understanding our chemical world
P. 340
INTRODUCING HALF-CELLS AND ELECTRODE POTENTIALS 307
Surface atom Positive ion
(cation)
− + +
−
Electrode Excess surface charge
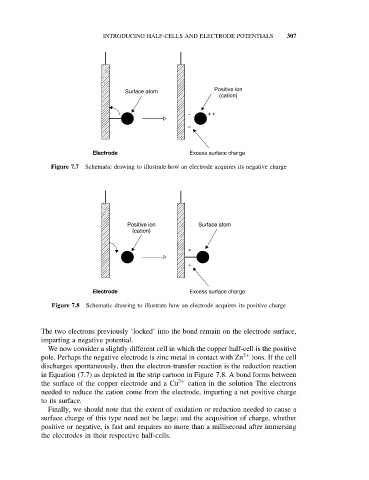
Figure 7.7 Schematic drawing to illustrate how an electrode acquires its negative charge
Positive ion Surface atom
(cation)
+
+
Electrode Excess surface charge
Figure 7.8 Schematic drawing to illustrate how an electrode acquires its positive charge
The two electrons previously ‘locked’ into the bond remain on the electrode surface,
imparting a negative potential.
We now consider a slightly different cell in which the copper half-cell is the positive
pole. Perhaps the negative electrode is zinc metal in contact with Zn 2+ ions. If the cell
discharges spontaneously, then the electron-transfer reaction is the reduction reaction
in Equation (7.7) as depicted in the strip cartoon in Figure 7.8. A bond forms between
the surface of the copper electrode and a Cu 2+ cation in the solution The electrons
needed to reduce the cation come from the electrode, imparting a net positive charge
to its surface.
Finally, we should note that the extent of oxidation or reduction needed to cause a
surface charge of this type need not be large; and the acquisition of charge, whether
positive or negative, is fast and requires no more than a millisecond after immersing
the electrodes in their respective half-cells.