Page 335 - Physical chemistry understanding our chemical world
P. 335
302 ELECTROCHEMISTRY
constituent parts, so Na(Hg) is solid and, when prepared with certain concentrations
of sodium, does not even react with water.
Why is electricity more dangerous in wet weather?
Electrolytes for cells, and introducing ions
Most electrical apparatus is safe when operated in a dry environment, but everyone
should know that water and electricity represent a lethal combination. Only a minimal
amount of charge conducts through air, so cutting dry grass with an electric mower
is safe. Cutting the same grass during a heavy downpour risks electrocution, because
water is a good conductor of electricity.
But water does not conduct electrons, so the charges must move
Currents conduct through water by a wholly different mechanism than through a
throughanelectrolyte metallic electrode. In fact, the charge carriers through solutions –
by means of ions. aqueous or otherwise – are solvated ions. The ‘mobility’ µ of an
ion in water is sufficiently high that charge conducts rapidly from
a wet electrical appliance toward the person holding it: it behaves
Ultimately, the word as an electrolyte.
‘ion’ derives from the
Greek eimi ‘to go’, All cells comprise half-cells, electrodes and a conductive elec-
implying the arrival of trolyte; the latter component separates the electrodes and conducts
someone or something. ions. It is usually, although not always, a liquid and normally has
We get the English an ionic substance dissolved within it, the solid dissociating in
word ‘aim’ from this solution to form ions. Aqueous electrolytes are a favourite choice
root. because the high ‘dielectric constant’ of water imparts a high
‘ionic conductivity’ κ to the solution.
Sometimes electrochemists are forced to construct electrochem-
Ionic conductivity is ical cells without water, e.g. if the analyte is water sensitive or
often given the Greek merely insoluble. In these cases, we construct the cell with an
symbol kappa (κ) organic solvent, the usual choice being the liquids acetonitrile,
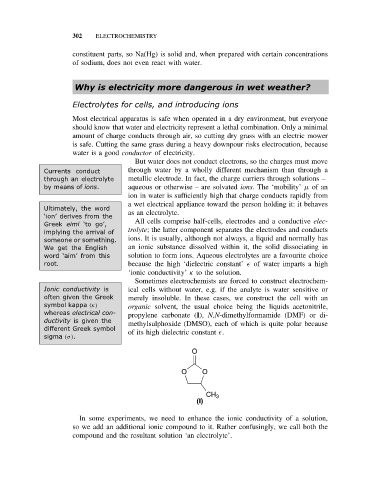
whereas electrical con- propylene carbonate (I), N,N-dimethylformamide (DMF) or di-
ductivity is given the methylsulphoxide (DMSO), each of which is quite polar because
different Greek symbol of its high dielectric constant .
sigma (σ).
O
O O
CH 3
(I)
In some experiments, we need to enhance the ionic conductivity of a solution,
so we add an additional ionic compound to it. Rather confusingly, we call both the
compound and the resultant solution ‘an electrolyte’.