Page 333 - Physical chemistry understanding our chemical world
P. 333
300 ELECTROCHEMISTRY
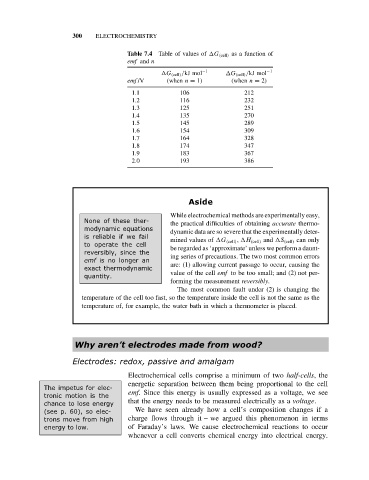
Table 7.4 Table of values of G (cell) as a function of
emf and n
G (cell) /kJ mol −1 G (cell) /kJ mol −1
emf /V (when n = 1) (when n = 2)
1.1 106 212
1.2 116 232
1.3 125 251
1.4 135 270
1.5 145 289
1.6 154 309
1.7 164 328
1.8 174 347
1.9 183 367
2.0 193 386
Aside
While electrochemical methods are experimentally easy,
None of these ther- the practical difficulties of obtaining accurate thermo-
modynamic equations dynamic data are so severe that the experimentally deter-
is reliable if we fail
mined values of G (cell) , H (cell) and S (cell) can only
to operate the cell be regarded as ‘approximate’ unless we perform a daunt-
reversibly, since the
ing series of precautions. The two most common errors
emf is no longer an
exact thermodynamic are: (1) allowing current passage to occur, causing the
value of the cell emf to be too small; and (2) not per-
quantity.
forming the measurement reversibly.
The most common fault under (2) is changing the
temperature of the cell too fast, so the temperature inside the cell is not the same as the
temperature of, for example, the water bath in which a thermometer is placed.
Why aren’t electrodes made from wood?
Electrodes: redox, passive and amalgam
Electrochemical cells comprise a minimum of two half-cells,the
energetic separation between them being proportional to the cell
The impetus for elec-
tronic motion is the emf. Since this energy is usually expressed as a voltage, we see
that the energy needs to be measured electrically as a voltage.
chance to lose energy
(see p. 60), so elec- We have seen already how a cell’s composition changes if a
trons move from high charge flows through it – we argued this phenomenon in terms
energy to low. of Faraday’s laws. We cause electrochemical reactions to occur
whenever a cell converts chemical energy into electrical energy.