Page 374 - Physical chemistry understanding our chemical world
P. 374
TRANSPORT PHENOMENA 341
+
Potential/V potential Rest potential
Time t/ms
Action
−
Onset of impulse
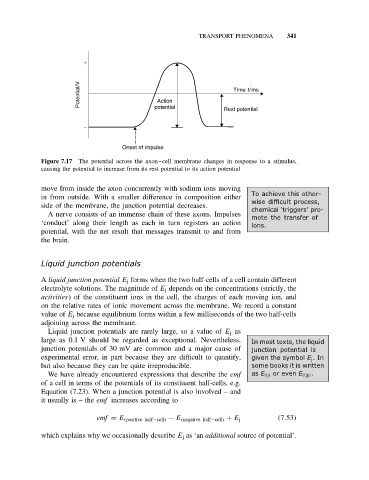
Figure 7.17 The potential across the axon–cell membrane changes in response to a stimulus,
causing the potential to increase from its rest potential to its action potential
move from inside the axon concurrently with sodium ions moving
To achieve this other-
in from outside. With a smaller difference in composition either
wise difficult process,
side of the membrane, the junction potential decreases.
chemical ‘triggers’ pro-
A nerve consists of an immense chain of these axons. Impulses
mote the transfer of
‘conduct’ along their length as each in turn registers an action ions.
potential, with the net result that messages transmit to and from
the brain.
Liquid junction potentials
A liquid junction potential E j forms when the two half-cells of a cell contain different
electrolyte solutions. The magnitude of E j depends on the concentrations (strictly, the
activities) of the constituent ions in the cell, the charges of each moving ion, and
on the relative rates of ionic movement across the membrane. We record a constant
value of E j because equilibrium forms within a few milliseconds of the two half-cells
adjoining across the membrane.
Liquid junction potentials are rarely large, so a value of E j as
large as 0.1 V should be regarded as exceptional. Nevertheless, In most texts, the liquid
junction potentials of 30 mV are common and a major cause of junction potential is
experimental error, in part because they are difficult to quantify, given the symbol E j .In
but also because they can be quite irreproducible. some books it is written
We have already encountered expressions that describe the emf as E (lj) or even E (ljp) .
of a cell in terms of the potentials of its constituent half-cells, e.g.
Equation (7.23). When a junction potential is also involved – and
it usually is – the emf increases according to
(7.53)
emf = E (positive half−cell) − E (negative half−cell) + E j
which explains why we occasionally describe E j as ‘an additional source of potential’.