Page 379 - Physical chemistry understanding our chemical world
P. 379
346 ELECTROCHEMISTRY
zinc were then immersed in their respective solutions. The electrode reaction at the
−
zinc anode is Zn → Zn 2+ + 2e , while reduction occurs at the positive electrode,
−
Cu 2+ + 2e → Cu.
Although this battery was efficient, it was never popular because it required aqueous
solutions, which can be a danger if they slopped about. Its market share also suffered
when better batteries were introduced to the market.
The Leclanch´ e ‘dry-cell’ battery
The Leclanch´ e cell was first sold in 1880, and is still probably the most popular
battery in the world today, being needed for everyday applications such as torches,
radios, etc. It delivers an emf of ca 1.6 V.
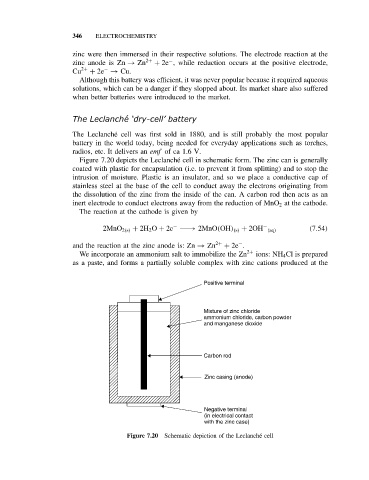
Figure 7.20 depicts the Leclanch´ e cell in schematic form. The zinc can is generally
coated with plastic for encapsulation (i.e. to prevent it from splitting) and to stop the
intrusion of moisture. Plastic is an insulator, and so we place a conductive cap of
stainless steel at the base of the cell to conduct away the electrons originating from
the dissolution of the zinc from the inside of the can. A carbon rod then acts as an
inert electrode to conduct electrons away from the reduction of MnO 2 at the cathode.
The reaction at the cathode is given by
− −
2MnO 2(s) + 2H 2 O + 2e −−→ 2MnO(OH) (s) + 2OH (aq) (7.54)
and the reaction at the zinc anode is: Zn → Zn 2+ + 2e .
−
We incorporate an ammonium salt to immobilize the Zn 2+ ions: NH 4 Cl is prepared
as a paste, and forms a partially soluble complex with zinc cations produced at the
Positive terminal
Mixture of zinc chloride
ammonium chloride, carbon powder
and manganese dioxide
Carbon rod
Zinc casing (anode)
Negative terminal
(in electrical contact
with the zinc case)
Figure 7.20 Schematic depiction of the Leclanch´ e cell