Page 105 - Physical chemistry eng
P. 105
82 CHAPTER 4 Thermochemistry
Q4.17 Under what conditions are ¢H and ¢U for a reac- Q4.19 Is ¢ H for breaking the first C—H bond in methane
tion involving gases and/or liquids or solids identical? equal to the average C—H bond enthalpy in this molecule?
Explain your answer.
Q4.18 Dogs cool off in hot weather by panting.
Write a chemical equation to describe this process and Q4.20 Humans cool off through perspiration. How does the
calculate ¢H° R . effectiveness of this process depend on the relative humidity?
Numerical Problems
Problem numbers in red indicate that the solution to the Calculate ¢H° R for
problem is given in the Student’s Solutions Manual. a. OH(g) ¡ H(g) + O(g)
P4.1 Given the data in Table 4.1 (Appendix B, Data Tables) b. H O(g) ¡ 2 H(g) + O(g)
2
and the following information, calculate the single bond
c. H O(g) ¡ H(g) + OH(g)
2
enthalpies and energies for Si–F, Si–Cl, C–F, N–F, O–F, H–F:
Assuming ideal gas behavior, calculate ¢H° and ¢U° R for all
R
Substance SiF (g) SiCl (g)CF (g) NF (g) OF (g) HF(g) three reactions.
4
4
4
2
3
-1
¢H° f (kJ mol ) -1614.9 -657.0 -925 -125 -22 -271 P4.9 Calculate the standard enthalpy of formation of FeS 2 (s)
P4.2 At 1000. K, ¢H° R = –123.77 kJ mol -1 for the reaction at 600.°C from the following data at 298.15 K. Assume that the
N (g) + 3 H (g) ¡ 2 NH (g), with C P,m = 3.502R, 3.466R, heat capacities are independent of temperature.
3
2
2
and 4.217R for N (g), H (g), and NH (g), respectively. Substance Fe(s) FeS (s) Fe O (s) S(rhombic) SO (g)
3
2
2
2
3
2
2
Calculate ¢H° f of NH 3 (g) at 450. K from this information. ¢H° (kJ mol ) –824.2 -296.81
–1
Assume that the heat capacities are independent of temperature. f
C > R 3.02 7.48 2.72
P4.3 A sample of K(s) of mass 2.740 g undergoes P,m
combustion in a constant volume calorimeter at 298.15 K. You are also given that for the reaction 2 FeS (s) + 11>2 O (g)
2
–1
The calorimeter constant is 1849 J K , and the measured ¡ Fe O (s) + 4 SO (g), ¢H° =-1655 kJ mol . 2
-1
temperature rise in the inner water bath containing 1450. g of 2 3 2 R
water is 1.60 K. Calculate ¢U° f and ¢H° f for K O. P4.10 The following data are a DSC scan of a solution of a
2
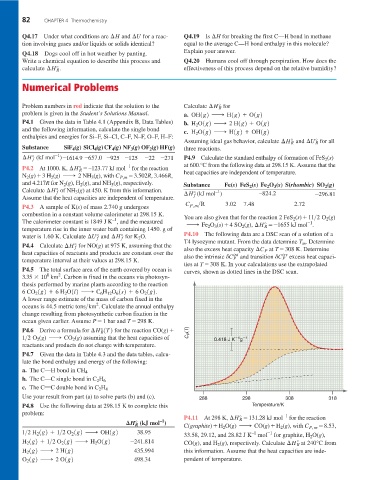
T4 lysozyme mutant. From the data determine T . Determine
m
P4.4 Calculate ¢H° f for NO(g) at 975 K, assuming that the also the excess heat capacity ¢C at T = 308 K. Determine
heat capacities of reactants and products are constant over the int P trs
also the intrinsic dC P and transition dC P excess heat capaci-
temperature interval at their values at 298.15 K.
ties at T = 308 K. In your calculations use the extrapolated
P4.5 The total surface area of the earth covered by ocean is curves, shown as dotted lines in the DSC scan.
8
2
3.35 * 10 km . Carbon is fixed in the oceans via photosyn-
thesis performed by marine plants according to the reaction
6 CO 2 (g) + 6 H 2 O(l) ¡ C 6 H 12 O 6 (s) + 6 O 2 (g) .
A lower range estimate of the mass of carbon fixed in the
>
2
oceans is 44.5 metric tons km . Calculate the annual enthalpy
change resulting from photosynthetic carbon fixation in the
ocean given earlier. Assume P = 1 bar and T = 298 K.
P4.6 Derive a formula for ¢H°(T) for the reaction CO(g) + C P (T)
R
1>2 O (g) ¡ CO (g) assuming that the heat capacities of 0.418 J K 1 1
2
2
g
reactants and products do not change with temperature.
P4.7 Given the data in Table 4.3 and the data tables, calcu-
late the bond enthalpy and energy of the following:
a. The C—H bond in CH 4
b. The C—C single bond in C H
2 6
c. The C—C double bond in C H
2 4
Use your result from part (a) to solve parts (b) and (c). 288 298 308 318
P4.8 Use the following data at 298.15 K to complete this Temperature/K
problem: -1
P4.11 At 298 K, ¢H° R = 131.28 kJ mol for the reaction
–1
≤H° R (kJ mol ) C(graphite) + H O(g) ¡ CO(g) + H (g), with C P, m = 8.53,
2
2
1>2 H 2 (g) + 1>2 O 2 (g) ¡ OH(g) 38.95 –1 -1
33.58, 29.12, and 28.82 J K mol for graphite, H O(g),
2
H 2 (g) + 1>2 O 2 (g) ¡ H 2 O(g) -241.814 CO(g), and H (g), respectively. Calculate ¢H R at 240°C from
2
H 2 (g) ¡ 2 H(g) 435.994 this information. Assume that the heat capacities are inde-
O 2 (g) ¡ 2 O(g) 498.34 pendent of temperature.