Page 29 - Physical chemistry eng
P. 29
6 CHAPTER 1 Fundamental Concepts of Thermodynamics
point, even for different gases. This suggests a unique reference point for temperature,
rather than the two reference points used in constructing the centigrade scale. The value
zero is given to the temperature at which P : 0 , so that a = 0 . However, this choice is
not sufficient to define the temperature scale, because the size of the degree is undefined.
By convention, the size of the degree on the absolute temperature scale is set equal to the
size of the degree on the Celsius scale. With these two choices, the absolute and Celsius
temperature scales are related by Equation (1.16). The scale measured by the ideal gas
thermometer is the absolute temperature scale used in thermodynamics. The unit of tem-
perature on this scale is called the kelvin, abbreviated K (without a degree sign):
T>K = T >°C + 273.15 (1.16)
C
Basic Definitions Needed to Describe
1.3 Thermodynamic Systems
Having discussed the macroscopic variables pressure, volume, and temperature, we
introduce some important concepts used in thermodynamics. A thermodynamic system
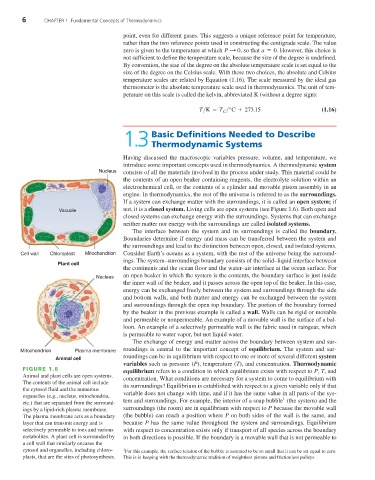
Nucleus consists of all the materials involved in the process under study. This material could be
the contents of an open beaker containing reagents, the electrolyte solution within an
electrochemical cell, or the contents of a cylinder and movable piston assembly in an
engine. In thermodynamics, the rest of the universe is referred to as the surroundings.
If a system can exchange matter with the surroundings, it is called an open system; if
Vacuole not, it is a closed system. Living cells are open systems (see Figure 1.6). Both open and
closed systems can exchange energy with the surroundings. Systems that can exchange
neither matter nor energy with the surroundings are called isolated systems.
The interface between the system and its surroundings is called the boundary.
Boundaries determine if energy and mass can be transferred between the system and
the surroundings and lead to the distinction between open, closed, and isolated systems.
Cell wall Chloroplast Mitochondrion Consider Earth’s oceans as a system, with the rest of the universe being the surround-
ings. The system–surroundings boundary consists of the solid–liquid interface between
Plant cell
the continents and the ocean floor and the water–air interface at the ocean surface. For
Nucleus an open beaker in which the system is the contents, the boundary surface is just inside
the inner wall of the beaker, and it passes across the open top of the beaker. In this case,
energy can be exchanged freely between the system and surroundings through the side
and bottom walls, and both matter and energy can be exchanged between the system
and surroundings through the open top boundary. The portion of the boundary formed
by the beaker in the previous example is called a wall. Walls can be rigid or movable
and permeable or nonpermeable. An example of a movable wall is the surface of a bal-
loon. An example of a selectively permeable wall is the fabric used in raingear, which
is permeable to water vapor, but not liquid water.
The exchange of energy and matter across the boundary between system and sur-
Mitochondrion Plasma membrane roundings is central to the important concept of equilibrium. The system and sur-
roundings can be in equilibrium with respect to one or more of several different system
Animal cell
variables such as pressure (P), temperature (T), and concentration. Thermodynamic
FIGURE 1.6
equilibrium refers to a condition in which equilibrium exists with respect to P, T, and
Animal and plant cells are open systems.
concentration. What conditions are necessary for a system to come to equilibrium with
The contents of the animal cell include
its surroundings? Equilibrium is established with respect to a given variable only if that
the cytosol fluid and the numerous
variable does not change with time, and if it has the same value in all parts of the sys-
organelles (e.g., nucleus, mitochondria, 1
tem and surroundings. For example, the interior of a soap bubble (the system) and the
etc.) that are separated from the surround-
ings by a lipid-rich plasma membrane. surroundings (the room) are in equilibrium with respect to P because the movable wall
The plasma membrane acts as a boundary (the bubble) can reach a position where P on both sides of the wall is the same, and
layer that can transmit energy and is because P has the same value throughout the system and surroundings. Equilibrium
selectively permeable to ions and various with respect to concentration exists only if transport of all species across the boundary
metabolites. A plant cell is surrounded by in both directions is possible. If the boundary is a movable wall that is not permeable to
a cell wall that similarly encases the
cytosol and organelles, including chloro- 1 For this example, the surface tension of the bubble is assumed to be so small that it can be set equal to zero.
plasts, that are the sites of photosynthesis. This is in keeping with the thermodynamic tradition of weightless pistons and frictionless pulleys.